Biogeochemical Cycles PPT
... processes of life come from the elements that were present in the Earth's crust when it was formed billions of years ago. This matter, the building blocks of life, continually cycles through Earth's systems, the atmosphere, hydrosphere, biosphere, and lithosphere, on time scales that range from a fe ...
... processes of life come from the elements that were present in the Earth's crust when it was formed billions of years ago. This matter, the building blocks of life, continually cycles through Earth's systems, the atmosphere, hydrosphere, biosphere, and lithosphere, on time scales that range from a fe ...
Ecological Cycles
... breakdown (too big) Accumulates undecomposed over time forming peat Peat over time and under pressure forms fossil fuels When fossil fuels are burned – releases carbon ...
... breakdown (too big) Accumulates undecomposed over time forming peat Peat over time and under pressure forms fossil fuels When fossil fuels are burned – releases carbon ...
Soil and Applied Sulfur (A2525)
... Authors: E.E. Schulte and K.A. Kelling are professors of soil science, College of Agricultural and Life Sciences, University of WisconsinMadison and University of Wisconsin-Extension, Cooperative Extension. The authors wish to thank L.M. Walsh, professor of soil science, University of Wisconsin-Madi ...
... Authors: E.E. Schulte and K.A. Kelling are professors of soil science, College of Agricultural and Life Sciences, University of WisconsinMadison and University of Wisconsin-Extension, Cooperative Extension. The authors wish to thank L.M. Walsh, professor of soil science, University of Wisconsin-Madi ...
I Must Have That Formula
... representation of how carbohydrates are broken down, or oxidized, thereby releasing energy for use by the consuming organisms. The carbon used and circulated in photosynthesis represents only a tiny portion of the available global carbon. Burning Coal: C ( s) O2 CO2 Burning Natural Gas: CH 4 2 ...
... representation of how carbohydrates are broken down, or oxidized, thereby releasing energy for use by the consuming organisms. The carbon used and circulated in photosynthesis represents only a tiny portion of the available global carbon. Burning Coal: C ( s) O2 CO2 Burning Natural Gas: CH 4 2 ...
Document
... oxygen atoms are examples of free radicals; they quickly enter into chemical reactions that allow them to attain stable arrangements of electrons. In the stratosphere free radicals can combine with oxygen molecules to form ozone. A third molecule, typically nitrogen gas or atmospheric oxygen (repres ...
... oxygen atoms are examples of free radicals; they quickly enter into chemical reactions that allow them to attain stable arrangements of electrons. In the stratosphere free radicals can combine with oxygen molecules to form ozone. A third molecule, typically nitrogen gas or atmospheric oxygen (repres ...
Big Formulas
... molecules, splitting them into individual oxygen atoms. These highly reactive oxygen atoms are examples of free radicals; they quickly enter into chemical reactions that allow them to attain stable arrangements of electrons. In the stratosphere free radicals can combine with oxygen molecules to form ...
... molecules, splitting them into individual oxygen atoms. These highly reactive oxygen atoms are examples of free radicals; they quickly enter into chemical reactions that allow them to attain stable arrangements of electrons. In the stratosphere free radicals can combine with oxygen molecules to form ...
The Carbon Cycle : The different forms and compounds in which
... CFC’s (chlorofluorocarbons) are highly stable molecules in the troposphere, however, high-energy UV photons in the stratosphere split chlorine radicals from CFC’s by breaking their C-Cl bond. The freed chlorine radicals are very reactive and can participate in a series of reaction that destroy ozone ...
... CFC’s (chlorofluorocarbons) are highly stable molecules in the troposphere, however, high-energy UV photons in the stratosphere split chlorine radicals from CFC’s by breaking their C-Cl bond. The freed chlorine radicals are very reactive and can participate in a series of reaction that destroy ozone ...
I Must Have That Formula APES Chemistry Review From Kelly A
... CFC’s (chlorofluorocarbons) are highly stable molecules in the troposphere, however, highenergy UV photons in the stratosphere split chlorine radicals from CFC’s by breaking their CCl bond. The freed chlorine radicals are very reactive and can participate in a series of reaction that destroy ozone b ...
... CFC’s (chlorofluorocarbons) are highly stable molecules in the troposphere, however, highenergy UV photons in the stratosphere split chlorine radicals from CFC’s by breaking their CCl bond. The freed chlorine radicals are very reactive and can participate in a series of reaction that destroy ozone b ...
Biochemistry of Sulfur
... Thiosulfate Oxidation: A tetrathionate-forming, membrane-bound thiosulfate:quinone oxidoreductase (TQO) was isolated from aerobically grown Ac. ambivalens cells. Optimal activity was observed at 85 ˚C and pH 5. The 102 kDa glycosylated holoenzyme had a α2β2 stoichiometry. Oxygen electrode measureme ...
... Thiosulfate Oxidation: A tetrathionate-forming, membrane-bound thiosulfate:quinone oxidoreductase (TQO) was isolated from aerobically grown Ac. ambivalens cells. Optimal activity was observed at 85 ˚C and pH 5. The 102 kDa glycosylated holoenzyme had a α2β2 stoichiometry. Oxygen electrode measureme ...
1. dia
... • If natural water is in contact with limestone, dolomite, the pH does not change → buffer effect. The living organisms are killed by the increased CO2 content • In case of week buffer effect (small Ca- and Mg-hydro carbonate content) the living organisms are killed by the decreased pH ...
... • If natural water is in contact with limestone, dolomite, the pH does not change → buffer effect. The living organisms are killed by the increased CO2 content • In case of week buffer effect (small Ca- and Mg-hydro carbonate content) the living organisms are killed by the decreased pH ...
Module 3.3-14 Potassium Sulfate
... Agricultural Use. Concentrations of K in soil are often too low to support healthy plant growth. Potassium is needed to complete many essential functions in plants, such as activating enzyme reactions, synthesizing proteins, forming starch and sugars, and regulating water flow in cells and leaves. P ...
... Agricultural Use. Concentrations of K in soil are often too low to support healthy plant growth. Potassium is needed to complete many essential functions in plants, such as activating enzyme reactions, synthesizing proteins, forming starch and sugars, and regulating water flow in cells and leaves. P ...
How did the life begin?
... • A. Half-life = length of time it takes for ½ of any sample to decay to its stable form • B. Compare C-14 to C-12 • C.When an organism dies – uptake of carbon stops • D. Existing C-14 still continues to decay • E. After 5,730 years, ½ remains • F. Works if organism is less than 60,000 years old ...
... • A. Half-life = length of time it takes for ½ of any sample to decay to its stable form • B. Compare C-14 to C-12 • C.When an organism dies – uptake of carbon stops • D. Existing C-14 still continues to decay • E. After 5,730 years, ½ remains • F. Works if organism is less than 60,000 years old ...
Putting the spotlight on organic sulfur
... upper-ocean microbial food web and have low concentrations in the water column (in the nanomole range per liter, compared with micromole per liter for bulk DOS as found by Ksionzek et al.). DMSP is produced by many eukaryotic phytoplankton species and some cyanobacteria (10, 11). Phytoplankton commi ...
... upper-ocean microbial food web and have low concentrations in the water column (in the nanomole range per liter, compared with micromole per liter for bulk DOS as found by Ksionzek et al.). DMSP is produced by many eukaryotic phytoplankton species and some cyanobacteria (10, 11). Phytoplankton commi ...
APES-Cycles-of
... DNA and ATP; all living things must have phosphorus P is found in rocks, and enters food webs when plants take up phosphorus found in soil Only one that doesn’t cycle through the atmosphere! (no gaseous form) P is a limiting factor for primary productivity because it isn’t easy for plants to acquire ...
... DNA and ATP; all living things must have phosphorus P is found in rocks, and enters food webs when plants take up phosphorus found in soil Only one that doesn’t cycle through the atmosphere! (no gaseous form) P is a limiting factor for primary productivity because it isn’t easy for plants to acquire ...
8/28 A brief introduction to biologically important elements and their
... oxidized and reduced sulfur are used as substrates with which to metabolize carbon by bacteria. In other organisms, sulfur groups occur in protein-cleaving enzymes, and make important transition metal complexes with V, Fe, Ni, Zn and Mo (as noted above). Sulfur bonds determine the higher structure ( ...
... oxidized and reduced sulfur are used as substrates with which to metabolize carbon by bacteria. In other organisms, sulfur groups occur in protein-cleaving enzymes, and make important transition metal complexes with V, Fe, Ni, Zn and Mo (as noted above). Sulfur bonds determine the higher structure ( ...
Wyatt Smith
... We need to allow an allotted amount of time for the soil to replenish itself with nutrients before we can even think about altering it in some form. (b) Explain why the restoration of the land would likely be more difficult in an arid climate (less than ten inches of precipitation per year). In an a ...
... We need to allow an allotted amount of time for the soil to replenish itself with nutrients before we can even think about altering it in some form. (b) Explain why the restoration of the land would likely be more difficult in an arid climate (less than ten inches of precipitation per year). In an a ...
Potassium Sulfate - International Plant Nutrition Institute
... Concentrations of K in soil are often too low to support healthy plant growth. Potassium is needed to complete many essential functions in plants, such as activating enzyme reactions, synthesizing proteins, forming starch and sugars, and regulating water flow in cells and leaves. Potassium sulfa ...
... Concentrations of K in soil are often too low to support healthy plant growth. Potassium is needed to complete many essential functions in plants, such as activating enzyme reactions, synthesizing proteins, forming starch and sugars, and regulating water flow in cells and leaves. Potassium sulfa ...
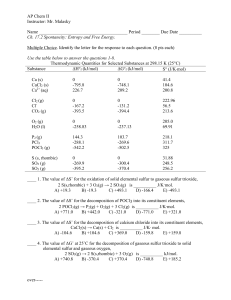
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
... ____ 5. The value of ΔG˚ at 25˚C for the decomposition of gaseous sulfur dioxide to solid elemental sulfur and gaseous oxygen, SO2(g) → 2 S (s,rhombic) + O2(g) is __________ kJ/mol. A) +395.2 B) +269.9 C) -269.9 D) +300.4 E) -300.4 ____ 6. The value of ΔG˚ at 25˚C for the formation of POCl3 from it ...
Nutritional Pattern Among Orgnaisms
... energy, cannot convert carbon dioxide to sugar; rather, they use organic compounds • Photoheterotrophs are the green nonsulfur bacteria, Chloroflexus, purple nonsulfur bacteria, Rhodopseudomonas ...
... energy, cannot convert carbon dioxide to sugar; rather, they use organic compounds • Photoheterotrophs are the green nonsulfur bacteria, Chloroflexus, purple nonsulfur bacteria, Rhodopseudomonas ...
NAME REVIEW 1: JUST THE BASICS ___1) In which material are
... 1) Oxidation occurs at the anode only 2) Reduction occurs at the anode only 3) Oxidation occurs at both the anode and the cathode 4) Reduction occurs at both the anode and the cathode ...
... 1) Oxidation occurs at the anode only 2) Reduction occurs at the anode only 3) Oxidation occurs at both the anode and the cathode 4) Reduction occurs at both the anode and the cathode ...
Forcing
... Anthropogenic Sulfate Aerosols Coal and diesel fuel contain sulfur Burning of these fuels produces sulfur dioxide (a gas) In the atmosphere, this gas is converted into particles ...
... Anthropogenic Sulfate Aerosols Coal and diesel fuel contain sulfur Burning of these fuels produces sulfur dioxide (a gas) In the atmosphere, this gas is converted into particles ...
Chapter 27
... • Nitrogen makes up ~ 80% of the Earth’s atmosphere – Exists as nitrogen gas (N2) ...
... • Nitrogen makes up ~ 80% of the Earth’s atmosphere – Exists as nitrogen gas (N2) ...
Balancing Reactions 1
... 5. Write balanced formula unit equations for the following redox reactions: a. Aluminum reacts with sulfuric acid, H2SO4, to produce aluminum sulfate and hydrogen. b. Nitrogen reacts with hydrogen to form ammonia, NH3 c. Zinc sulfide, ZnS, reacts with oxygen to from zinc oxide and sulfur dioxide ...
... 5. Write balanced formula unit equations for the following redox reactions: a. Aluminum reacts with sulfuric acid, H2SO4, to produce aluminum sulfate and hydrogen. b. Nitrogen reacts with hydrogen to form ammonia, NH3 c. Zinc sulfide, ZnS, reacts with oxygen to from zinc oxide and sulfur dioxide ...
arsenic removal by controlled biological iron oxidation reactions
... sulfate from aqueous streams. These technologies find their origin in the exploration of microorganisms involved in the global sulfur cycle. Currently, several sulfur cycle biotechnologies are applied successfully at full-scale. The sulfur cycle is closely linked with the iron cycle, and also the la ...
... sulfate from aqueous streams. These technologies find their origin in the exploration of microorganisms involved in the global sulfur cycle. Currently, several sulfur cycle biotechnologies are applied successfully at full-scale. The sulfur cycle is closely linked with the iron cycle, and also the la ...
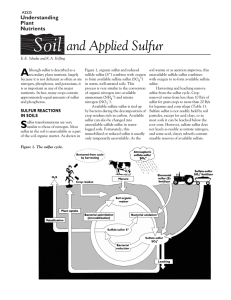
Sulfur cycle

The sulfur cycle is the collection of processes by which sulfur moves to and from minerals (including the waterways) and living systems. Such biogeochemical cycles are important in geology because they affect many minerals. Biogeochemical cycles are also important for life because sulfur is an essential element, being a constituent of many proteins and cofactors.Steps of the sulfur cycle are: Mineralization of organic sulfur into inorganic forms, such as hydrogen sulfide (H2S), elemental sulfur, as well as sulfide minerals. Oxidation of hydrogen sulfide, sulfide, and elemental sulfur (S) to sulfate (SO42−). Reduction of sulfate to sulfide. Incorporation of sulfide into organic compounds (including metal-containing derivatives).These are often termed as follows:Assimilative sulfate reduction (see also sulfur assimilation) in which sulfate (SO42−) is reduced by plants, fungi and various prokaryotes. The oxidation states of sulfur are +6 in sulfate and –2 in R–SH.Desulfurization in which organic molecules containing sulfur can be desulfurized, producing hydrogen sulfide gas (H2S, oxidation state = –2). An analogous process for organic nitrogen compounds is deamination.Oxidation of hydrogen sulfide produces elemental sulfur (S8), oxidation state = 0. This reaction occurs in the photosynthetic green and purple sulfur bacteria and some chemolithotrophs. Often the elemental sulfur is stored as polysulfides.Oxidation of elemental sulfur by sulfur oxidizers produces sulfate.Dissimilative sulfur reduction in which elemental sulfur can be reduced to hydrogen sulfide.Dissimilative sulfate reduction in which sulfate reducers generate hydrogen sulfide from sulfate.↑