Cellular Respiration
... Glycolysis: • Occurs in the cytoplasm (cytosol) • Chemical RXN where sugarGLUCOSE is broken down into 2 Pyruvic Acid molecules • Produces 2 ATP molecules for every ...
... Glycolysis: • Occurs in the cytoplasm (cytosol) • Chemical RXN where sugarGLUCOSE is broken down into 2 Pyruvic Acid molecules • Produces 2 ATP molecules for every ...
NOTES: 9.1-9.2 - Cellular Respiration
... How is the energy in sugar (glucose) molecules released so it can be used by the cells of an organism? ● Cellular Respiration occurs in both plant & animal cells -WHERE? ...
... How is the energy in sugar (glucose) molecules released so it can be used by the cells of an organism? ● Cellular Respiration occurs in both plant & animal cells -WHERE? ...
Unique plant respiration
... • 2) Citric Acid Cycle (aka Krebs or TCA cycle) – Pyruvate converted to CO2 and electrons • 3) Electron transport chain – Electrons reduce O2 to H20 and create ATP Energy storage • Plants store energy in the form of the carbohydrates sucrose and starch • In stroma of chloroplast, enzymes such as -am ...
... • 2) Citric Acid Cycle (aka Krebs or TCA cycle) – Pyruvate converted to CO2 and electrons • 3) Electron transport chain – Electrons reduce O2 to H20 and create ATP Energy storage • Plants store energy in the form of the carbohydrates sucrose and starch • In stroma of chloroplast, enzymes such as -am ...
Metabolism 2.0 - Max-Planck
... converted into sugar molecules. The cycle uses ATP as an energy source. The enzyme RuBisCO makes it possible to convert carbon dioxide to the sugar ribulose-1,5-bisphosphate (carbon dioxide fixation). The cycle must run three times, fixing three carbon dioxide molecules to produce one molecule of th ...
... converted into sugar molecules. The cycle uses ATP as an energy source. The enzyme RuBisCO makes it possible to convert carbon dioxide to the sugar ribulose-1,5-bisphosphate (carbon dioxide fixation). The cycle must run three times, fixing three carbon dioxide molecules to produce one molecule of th ...
What are macromolecules?
... 2. antibodies--to help fight disease 3. enzymes—speed up reaction in both plants and animals Formed from the bonding of monomer ...
... 2. antibodies--to help fight disease 3. enzymes—speed up reaction in both plants and animals Formed from the bonding of monomer ...
Ch 7 outline
... Where Is the Energy in Food? (p. 138; Figs. 7.1, 7.2, 7.3) A. Plants store energy from sunlight in the form of organic compounds, but all other organisms, plants included, must oxidize the organic compounds, using a process known as cellular respiration, to supply the energy needed to drive cellular ...
... Where Is the Energy in Food? (p. 138; Figs. 7.1, 7.2, 7.3) A. Plants store energy from sunlight in the form of organic compounds, but all other organisms, plants included, must oxidize the organic compounds, using a process known as cellular respiration, to supply the energy needed to drive cellular ...
Kreb`s Cycle
... Why do living things need food? • provides living things • source of energy with the chemical building blocks they need to grow and reproduce. • Source of raw materials for making new molecules ...
... Why do living things need food? • provides living things • source of energy with the chemical building blocks they need to grow and reproduce. • Source of raw materials for making new molecules ...
Function - berkeleyscience
... Photosynthesis in leaves, phloem in vascular system carries sugar-rich sap from leaves to where it’s needed ...
... Photosynthesis in leaves, phloem in vascular system carries sugar-rich sap from leaves to where it’s needed ...
Document
... c. Electrons fall to lower energy levels as they are passed down the chain (releases energy) d. Oxygen is the final electron acceptor e. The negative oxygen binds to 2 H+ to form water f. Chemiosmosis (see figure 2-13) i. The energy released by electrons moving down the chain is used to pump H+ from ...
... c. Electrons fall to lower energy levels as they are passed down the chain (releases energy) d. Oxygen is the final electron acceptor e. The negative oxygen binds to 2 H+ to form water f. Chemiosmosis (see figure 2-13) i. The energy released by electrons moving down the chain is used to pump H+ from ...
Cell Metabolism
... c. Electrons fall to lower energy levels as they are passed down the chain (releases energy) d. Oxygen is the final electron acceptor e. The negative oxygen binds to 2 H+ to form water f. Chemiosmosis (see figure 2-13) i. The energy released by electrons moving down the chain is used to pump H+ from ...
... c. Electrons fall to lower energy levels as they are passed down the chain (releases energy) d. Oxygen is the final electron acceptor e. The negative oxygen binds to 2 H+ to form water f. Chemiosmosis (see figure 2-13) i. The energy released by electrons moving down the chain is used to pump H+ from ...
Document
... + water + energy glucose + oxygen dioxide 6CO2 + 6H2O + light C6H12O6 + 6O2 energy Regents Biology ...
... + water + energy glucose + oxygen dioxide 6CO2 + 6H2O + light C6H12O6 + 6O2 energy Regents Biology ...
List and tell the function of the parts of a cell
... 14. What organelle in a plant cell is responsible for capturing light for photosynthesis? Chloroplast What organelle in plant and animal cells is responsible for breaking down sugars into ATP? Mitochondria 15. Why do bacteria and yeast have to go through fermentation to get energy rather than aerobi ...
... 14. What organelle in a plant cell is responsible for capturing light for photosynthesis? Chloroplast What organelle in plant and animal cells is responsible for breaking down sugars into ATP? Mitochondria 15. Why do bacteria and yeast have to go through fermentation to get energy rather than aerobi ...
File
... • Amino acids – are the small molecular units that make up the very large protein molecules a. 22 different amino acids b. 9 essential amino acids – must be ingested because they cannot be made by the body Enzymes • Specialized protein molecules found in all living cells • Help control chemical reac ...
... • Amino acids – are the small molecular units that make up the very large protein molecules a. 22 different amino acids b. 9 essential amino acids – must be ingested because they cannot be made by the body Enzymes • Specialized protein molecules found in all living cells • Help control chemical reac ...
Chapter 6, Section 3
... Organic: contains carbon ◦ All living things contain carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P) Monomer: created when C,H,O, N, P bond together to form small molecules Polymer: large compounds that are formed by joining monomers together ...
... Organic: contains carbon ◦ All living things contain carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P) Monomer: created when C,H,O, N, P bond together to form small molecules Polymer: large compounds that are formed by joining monomers together ...
Understanding Biochemistry
... up the basic structure, or “backbone,” of these compounds. Each atom of carbon has four electrons in its outer energy level, which makes it possible for each carbon atom to form four bonds with other atoms. • As a result, carbon atoms can form long chains. A huge number of different carbon compounds ...
... up the basic structure, or “backbone,” of these compounds. Each atom of carbon has four electrons in its outer energy level, which makes it possible for each carbon atom to form four bonds with other atoms. • As a result, carbon atoms can form long chains. A huge number of different carbon compounds ...
Bio Sem I review
... 2. Why is the cell membrane selectively permeable? 3. Discuss the conditions needed to cause water to diffuse into a cell. 4. Discuss the conditions necessary to cause water to diffuse out of a cell. 5. Describe what would happen to a red blood cell in each of the following situations: a. It is plac ...
... 2. Why is the cell membrane selectively permeable? 3. Discuss the conditions needed to cause water to diffuse into a cell. 4. Discuss the conditions necessary to cause water to diffuse out of a cell. 5. Describe what would happen to a red blood cell in each of the following situations: a. It is plac ...
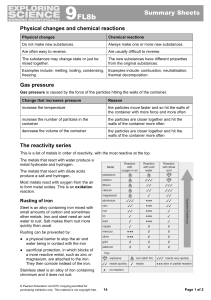
9F Reactivity - Parrs Wood High School
... This is a list of metals in order of reactivity, with the most reactive at the top. The metals that react with water produce a metal hydroxide and hydrogen. The metals that react with dilute acids produce a salt and hydrogen. Most metals react with oxygen from the air to form metal oxides. This is a ...
... This is a list of metals in order of reactivity, with the most reactive at the top. The metals that react with water produce a metal hydroxide and hydrogen. The metals that react with dilute acids produce a salt and hydrogen. Most metals react with oxygen from the air to form metal oxides. This is a ...
Cellular respiration *vs
... • Our cells use ATP as the energy required so they can do their work. This allows the body to function smoothly---to do work like: breathe, circulate blood, digest, respond to stimuli, create new cells, repair and grow, move our muscles, etc.,---everything you do uses energy. ...
... • Our cells use ATP as the energy required so they can do their work. This allows the body to function smoothly---to do work like: breathe, circulate blood, digest, respond to stimuli, create new cells, repair and grow, move our muscles, etc.,---everything you do uses energy. ...
4 Cell Resp Part 2 NT
... yields ____________________________________ only in presence of O2 (__________________________________________) ...
... yields ____________________________________ only in presence of O2 (__________________________________________) ...
Life Functions – Literacy Chart Vocabulary Term Book/internet
... Self-sustaining or self-nourishing organisms that have the ability to synthesize their own food from inorganic materials, e.g. carbon dioxide and nitrogen. ...
... Self-sustaining or self-nourishing organisms that have the ability to synthesize their own food from inorganic materials, e.g. carbon dioxide and nitrogen. ...
Photosynthesis

Photosynthesis is a process used by plants and other organisms to convert light energy, normally from the Sun, into chemical energy that can be later released to fuel the organisms' activities. This chemical energy is stored in carbohydrate molecules, such as sugars, which are synthesized from carbon dioxide and water – hence the name photosynthesis, from the Greek φῶς, phōs, ""light"", and σύνθεσις, synthesis, ""putting together"". In most cases, oxygen is also released as a waste product. Most plants, most algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis maintains atmospheric oxygen levels and supplies all of the organic compounds and most of the energy necessary for life on Earth.Although photosynthesis is performed differently by different species, the process always begins when energy from light is absorbed by proteins called reaction centres that contain green chlorophyll pigments. In plants, these proteins are held inside organelles called chloroplasts, which are most abundant in leaf cells, while in bacteria they are embedded in the plasma membrane. In these light-dependent reactions, some energy is used to strip electrons from suitable substances, such as water, producing oxygen gas. Furthermore, two further compounds are generated: reduced nicotinamide adenine dinucleotide phosphate (NADPH) and adenosine triphosphate (ATP), the ""energy currency"" of cells.In plants, algae and cyanobacteria, sugars are produced by a subsequent sequence of light-independent reactions called the Calvin cycle, but some bacteria use different mechanisms, such as the reverse Krebs cycle. In the Calvin cycle, atmospheric carbon dioxide is incorporated into already existing organic carbon compounds, such as ribulose bisphosphate (RuBP). Using the ATP and NADPH produced by the light-dependent reactions, the resulting compounds are then reduced and removed to form further carbohydrates, such as glucose.The first photosynthetic organisms probably evolved early in the evolutionary history of life and most likely used reducing agents, such as hydrogen or hydrogen sulfide, as sources of electrons, rather than water. Cyanobacteria appeared later; the excess oxygen they produced contributed to the oxygen catastrophe, which rendered the evolution of complex life possible. Today, the average rate of energy capture by photosynthesis globally is approximately 130 terawatts, which is about three times the current power consumption of human civilization.Photosynthetic organisms also convert around 100–115 thousand million metric tonnes of carbon into biomass per year.