EnviroRegulationofMicrobialMetabolism-rev
... phosphate bonds from ATP), reducing power, and sources of nitrogen, sulfur, and single carbon units. (C.) 12 precursor metabolites (D.) Role of the 12 precursors as a “pool” linking catabolism and anabolism. ATP, reduced pyridine nucleotide, and C1 units are also provided from catabolism to build th ...
... phosphate bonds from ATP), reducing power, and sources of nitrogen, sulfur, and single carbon units. (C.) 12 precursor metabolites (D.) Role of the 12 precursors as a “pool” linking catabolism and anabolism. ATP, reduced pyridine nucleotide, and C1 units are also provided from catabolism to build th ...
Chapter 9. Cellular Respiration STAGE 1: Glycolysis
... Cellular Respiration (harvesting ATP from glucose) in the absence of Oxygen. ...
... Cellular Respiration (harvesting ATP from glucose) in the absence of Oxygen. ...
Week 3 Notes
... Ferric iron (Fe3+) reduction to ferrous iron (Fe2+) Relatively large positive Eo’ indicates that Fe3+ is an attractive electron acceptor Ferrous iron is much more soluble and this process has been used in mining iron ore Because of the high concentrations of iron in some groundwaters, iron reduction ...
... Ferric iron (Fe3+) reduction to ferrous iron (Fe2+) Relatively large positive Eo’ indicates that Fe3+ is an attractive electron acceptor Ferrous iron is much more soluble and this process has been used in mining iron ore Because of the high concentrations of iron in some groundwaters, iron reduction ...
role of respiration in glycolysis, co2 and h20 production
... Set of the metabolic reactions that occur in cells to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions that involve the oxidation of one molecule and the reduction of another. ...
... Set of the metabolic reactions that occur in cells to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions that involve the oxidation of one molecule and the reduction of another. ...
Photosynthesis and Cellular Respiration Vocabulary File
... Occurs in the inner membrane of the mitochondria Starting molecules: NADH and FADH2 and oxygen o Uses the NADH and FADH2 from the Kreb’s Cycle and another NADH from Glycolysis. Produces: Water and 32 ATP’s o FADH2 and NADH, release H’s so they can attach to oxygen and produce water 15) NADH & ...
... Occurs in the inner membrane of the mitochondria Starting molecules: NADH and FADH2 and oxygen o Uses the NADH and FADH2 from the Kreb’s Cycle and another NADH from Glycolysis. Produces: Water and 32 ATP’s o FADH2 and NADH, release H’s so they can attach to oxygen and produce water 15) NADH & ...
Ch 6 Notes
... organisms have carotenoids that remove the excess energy of singlet oxygen 2. Superoxide radicals – some form during incomplete reduction of oxygen in aerobic and anaerobic respiration – So reactive that aerobes produce superoxide dismutases to detoxify them – Anaerobes lack superoxide dismutase and ...
... organisms have carotenoids that remove the excess energy of singlet oxygen 2. Superoxide radicals – some form during incomplete reduction of oxygen in aerobic and anaerobic respiration – So reactive that aerobes produce superoxide dismutases to detoxify them – Anaerobes lack superoxide dismutase and ...
Ecology PowerPoint
... Commensalism = is an ecological relationship in which one species _____ and the other is neither _____ nor _____. ...
... Commensalism = is an ecological relationship in which one species _____ and the other is neither _____ nor _____. ...
3. Related Pathways
... converting the group to ammonia, NH3 (urea is expelled from the body in urine) Lipid Catabolism Glycerol can be converted to glucose through a process called gluconeogenesis Fatty acids undergo beta-oxidation where 2-C acetyl groups are removed Fats can produce 20% more ATP than carbohydrates ...
... converting the group to ammonia, NH3 (urea is expelled from the body in urine) Lipid Catabolism Glycerol can be converted to glucose through a process called gluconeogenesis Fatty acids undergo beta-oxidation where 2-C acetyl groups are removed Fats can produce 20% more ATP than carbohydrates ...
ATP GENERATION The energy captured within ATP can then be
... The energy captured within ATP can then be harnessed to create order in the form of biosynthetic reactions. In a hypothetical enzyme reaction that converts substrates A−H and B−OH to A−B and H2 O, the energy from ATP hydrolysis is first used to convert B−OH to a higher-energy intermediate, B−O−PO4. ...
... The energy captured within ATP can then be harnessed to create order in the form of biosynthetic reactions. In a hypothetical enzyme reaction that converts substrates A−H and B−OH to A−B and H2 O, the energy from ATP hydrolysis is first used to convert B−OH to a higher-energy intermediate, B−O−PO4. ...
Chapter 1 Homework - due Tuesday, Sept
... the production of ATP from ADP and Pi is catalyzed, and oxygen is reduced, forming water 4. What are the roles of NAD+ and FAD in aerobic respiration? NAD+ and FAD receive electrons at varying steps during glycolysis (NAD+ only) and the citric acid cycle (both NAD+ and FAD), to form NADH and FADH2, ...
... the production of ATP from ADP and Pi is catalyzed, and oxygen is reduced, forming water 4. What are the roles of NAD+ and FAD in aerobic respiration? NAD+ and FAD receive electrons at varying steps during glycolysis (NAD+ only) and the citric acid cycle (both NAD+ and FAD), to form NADH and FADH2, ...
Carbon Compounds
... Contain Carbon, Hydrogen, Oxygen, and Nitrogen Made of monomers called AMINO ACIDS Amino Acids are joined by peptide bonds, therefore another name for a protein is POLYPEPTIDE There are 4 levels of structural organization ...
... Contain Carbon, Hydrogen, Oxygen, and Nitrogen Made of monomers called AMINO ACIDS Amino Acids are joined by peptide bonds, therefore another name for a protein is POLYPEPTIDE There are 4 levels of structural organization ...
Learning Objectives
... What is the chemiosmotic model? Where are protons pumped? Through which complexes are protons pumped? What is the relationship between the number of electrons passed through the electron transport chain and the number of hydrogen ions pumped? What is a proton gradient? How is the proton gradient use ...
... What is the chemiosmotic model? Where are protons pumped? Through which complexes are protons pumped? What is the relationship between the number of electrons passed through the electron transport chain and the number of hydrogen ions pumped? What is a proton gradient? How is the proton gradient use ...
Biology Syllabus - Gull Lake Community Schools
... steady while external conditions may fluctuate. Human body 98.6 F or 37 C Blood pressure, blood sugar, heart rate… Single celled and multicelled organisms must regulate their internal situations. ...
... steady while external conditions may fluctuate. Human body 98.6 F or 37 C Blood pressure, blood sugar, heart rate… Single celled and multicelled organisms must regulate their internal situations. ...
External sources of energy → biologically energy : ATP
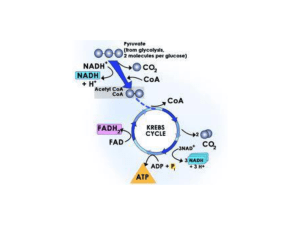
... C6H12O6 + 2NAD+ + 2ADP3- + 2Pi2- 2 C3H4O3 + 2NADH + 2 ATP4• Citric acid cycle • In mitochondrion • Pyruvate CO2 + NADH + FADH2 • Electron transport chain • High energy electrons from NADH and FADH2 O2 • Convert energy released into a proton motive force (H+ gradient) ...
... C6H12O6 + 2NAD+ + 2ADP3- + 2Pi2- 2 C3H4O3 + 2NADH + 2 ATP4• Citric acid cycle • In mitochondrion • Pyruvate CO2 + NADH + FADH2 • Electron transport chain • High energy electrons from NADH and FADH2 O2 • Convert energy released into a proton motive force (H+ gradient) ...
Science, Matter, Energy, and Systems Key Terms
... photosynthesis. Carbon is then passed into the food chain and returned to the atmosphere by the respiration and decay of animals, plants, and other organisms. The burning of fossil fuels also releases c ...
... photosynthesis. Carbon is then passed into the food chain and returned to the atmosphere by the respiration and decay of animals, plants, and other organisms. The burning of fossil fuels also releases c ...
FINAL EXAM - 09 December 2005
... This reaction is coupled to the oxidation of FADH2 to FAD+ This reaction is coupled to the oxidation of NADH + H+ to NAD+ This reaction is coupled to the formation of ATP This reaction is coupled to the reduction of NAD+ to NADH + H+ ...
... This reaction is coupled to the oxidation of FADH2 to FAD+ This reaction is coupled to the oxidation of NADH + H+ to NAD+ This reaction is coupled to the formation of ATP This reaction is coupled to the reduction of NAD+ to NADH + H+ ...
Topics
... electron carriers • NADH generates 3 ATP, FADH2 = 2 ATP • Membrane bound carriers transfer electrons (redox reactions) • The final electron acceptor completes the terminal step (ex. Oxygen) • Chemiosmosis • Proton motive force (PMF) ...
... electron carriers • NADH generates 3 ATP, FADH2 = 2 ATP • Membrane bound carriers transfer electrons (redox reactions) • The final electron acceptor completes the terminal step (ex. Oxygen) • Chemiosmosis • Proton motive force (PMF) ...
4 basic needs
... Living things need oxygen to release energy from food. Organisms living on land get oxygen from the air. Organisms living in water get oxygen from the water. Green plants, algae and some bacteria need both oxygen and carbon dioxide. Photosynthesis is the process by which green organisms turn the ene ...
... Living things need oxygen to release energy from food. Organisms living on land get oxygen from the air. Organisms living in water get oxygen from the water. Green plants, algae and some bacteria need both oxygen and carbon dioxide. Photosynthesis is the process by which green organisms turn the ene ...
Ecological succession
... This water vapor eventually condenses to form clouds, and is returned to the earth as precipitation. This process is called the water cycle. The processes of cell respiration and excretion also release some water to the environment as well. ...
... This water vapor eventually condenses to form clouds, and is returned to the earth as precipitation. This process is called the water cycle. The processes of cell respiration and excretion also release some water to the environment as well. ...
ANAEROBIC NITROGEN FIXERS ON MARS. B. G. Lewis, Dept. of
... from interpretation of the Viking lander lifedetection experiments) may serve as oxidants, albeit rather strong ones. These conditions, and effects on N2-fixation can be tested experimentally in laboratory microcosms. Another crucial component for survival of anaerobes is a carbon source. For Desulf ...
... from interpretation of the Viking lander lifedetection experiments) may serve as oxidants, albeit rather strong ones. These conditions, and effects on N2-fixation can be tested experimentally in laboratory microcosms. Another crucial component for survival of anaerobes is a carbon source. For Desulf ...
Ecology
... is lost to heat from one level to the next. Only 10% of your food is actually incorporated into making you! ...
... is lost to heat from one level to the next. Only 10% of your food is actually incorporated into making you! ...
METABOLIC COMPARTMENTATION
... The inner mitochondrial membrane is impermeable to NADH Electrons from NADH in the cytosol are transferred by electron shuttles. In the glycerol phosphate shuttle, NADH in the cytosol is used to reduce dihydoxyacetone phosphate in the reaction catalyzed by cytosolic glycerol 3-phosphate dehydrogenas ...
... The inner mitochondrial membrane is impermeable to NADH Electrons from NADH in the cytosol are transferred by electron shuttles. In the glycerol phosphate shuttle, NADH in the cytosol is used to reduce dihydoxyacetone phosphate in the reaction catalyzed by cytosolic glycerol 3-phosphate dehydrogenas ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)