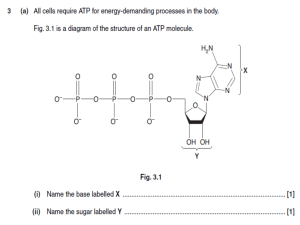
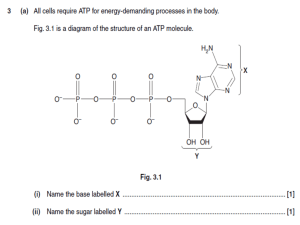
presentation source
... electron-transport chain to the next in a series of coupled oxidation-reduction reactions. B. As each cytochrome iron gains an electron, it becomes reduced; as it passes the electron to the next cytochrome, it becomes oxidized. C. The last cytochrome becomes oxidized by donating its electron to oxyg ...
... electron-transport chain to the next in a series of coupled oxidation-reduction reactions. B. As each cytochrome iron gains an electron, it becomes reduced; as it passes the electron to the next cytochrome, it becomes oxidized. C. The last cytochrome becomes oxidized by donating its electron to oxyg ...
8 Aerobic Respiration
... Aerobic respiration is the next step after Glycolysis if the cell can ...
... Aerobic respiration is the next step after Glycolysis if the cell can ...
Chapter 25
... 2 NADH produced during glycolysis produce 4-6 ATP 2 NADH produced during Acetyl CoA formation also produce 6 ATP 2 ATP from glycolysis ...
... 2 NADH produced during glycolysis produce 4-6 ATP 2 NADH produced during Acetyl CoA formation also produce 6 ATP 2 ATP from glycolysis ...
Anaerobic Respiration
... • Anaerobic conditions mean that there is no final hydrogen acceptor at the end of chemiosmosis. • Because there is no oxygen, NAD and FAD are not regenerated, which results in oxidation being blocked (NAD and FAD can’t get rid of H). • This subsequently means that no further link reaction, Krebs cy ...
... • Anaerobic conditions mean that there is no final hydrogen acceptor at the end of chemiosmosis. • Because there is no oxygen, NAD and FAD are not regenerated, which results in oxidation being blocked (NAD and FAD can’t get rid of H). • This subsequently means that no further link reaction, Krebs cy ...
Anaerobic Respiration
... • Anaerobic conditions mean that there is no final hydrogen acceptor at the end of chemiosmosis. • Because there is no oxygen, NAD and FAD are not regenerated, which results in oxidation being blocked (NAD and FAD can’t get rid of H). • This subsequently means that no further link reaction, Krebs cy ...
... • Anaerobic conditions mean that there is no final hydrogen acceptor at the end of chemiosmosis. • Because there is no oxygen, NAD and FAD are not regenerated, which results in oxidation being blocked (NAD and FAD can’t get rid of H). • This subsequently means that no further link reaction, Krebs cy ...
Document
... A. Sun—source of energy that fuels most life on Earth 1. Producers/Autotrophs—organisms that use an outside energy source to make energy-rich molecules a. Most producers use the Sun and contain chlorophyll, a chemical required for photosynthesis. b. Some producers, found near volcanic vents on the o ...
... A. Sun—source of energy that fuels most life on Earth 1. Producers/Autotrophs—organisms that use an outside energy source to make energy-rich molecules a. Most producers use the Sun and contain chlorophyll, a chemical required for photosynthesis. b. Some producers, found near volcanic vents on the o ...
Lab 11
... Discriminates organisms that can produce citrase to metabolize citrate into oxaloacetate and pyruvate. These organisms are forced to utilize ammonium salts as the nitrogen source producing alkaline ammonia waste. Results: Prussian blue slant and or butt = positive for _ ...
... Discriminates organisms that can produce citrase to metabolize citrate into oxaloacetate and pyruvate. These organisms are forced to utilize ammonium salts as the nitrogen source producing alkaline ammonia waste. Results: Prussian blue slant and or butt = positive for _ ...
Week 5 - UW Canvas
... d. is produced during the Krebs cycle. 5. The oxidation of glucose to CO2 and H2O… a. is exergonic. b. takes place entirely in the mitochondria. c. requires the electron transport chain. d. generates a pH gradient across the inner mitochondrial membrane in eukaryotes. e. only occurs in eukaryotes. 6 ...
... d. is produced during the Krebs cycle. 5. The oxidation of glucose to CO2 and H2O… a. is exergonic. b. takes place entirely in the mitochondria. c. requires the electron transport chain. d. generates a pH gradient across the inner mitochondrial membrane in eukaryotes. e. only occurs in eukaryotes. 6 ...
cellular respiration - Aurora City Schools
... the gain of oxygen) and reduction (the gaining of an electron, or hydrogen or losing oxygen by an element) ...
... the gain of oxygen) and reduction (the gaining of an electron, or hydrogen or losing oxygen by an element) ...
Life on Earth
... I can describe the two types of competition. I can describe pyramids of numbers/energy/biomass. ...
... I can describe the two types of competition. I can describe pyramids of numbers/energy/biomass. ...
apes final exam fall 09
... 3.If the world's population grew by 2% in 1998 and continued at that rate, how long would it take Earth's population to double? 4.You are visiting a developing country. Compared to a developed country, what types of differences would you expect to find? 5. Describe the second law of thermodynamics. ...
... 3.If the world's population grew by 2% in 1998 and continued at that rate, how long would it take Earth's population to double? 4.You are visiting a developing country. Compared to a developed country, what types of differences would you expect to find? 5. Describe the second law of thermodynamics. ...
ecology-unit-test-review-2016
... 11) Nutrients - chemical elements used by organisms to build and operate their bodies. example: carbon (C), oxygen (O), hydrogen (H), nitrogen (N) 12) Nutrient Cycles - movement of nutrients through the environment. ...
... 11) Nutrients - chemical elements used by organisms to build and operate their bodies. example: carbon (C), oxygen (O), hydrogen (H), nitrogen (N) 12) Nutrient Cycles - movement of nutrients through the environment. ...
Bio392 - Chapter 2-3 - notes
... body may need? • 3. How do you think your body used each of the foods that you ate? • 4. A common saying is “You are what you eat.” What do you think this statement means? ...
... body may need? • 3. How do you think your body used each of the foods that you ate? • 4. A common saying is “You are what you eat.” What do you think this statement means? ...
Ecology
... environment can support over a long period of time Important for conservationists in managing wildlife pops ...
... environment can support over a long period of time Important for conservationists in managing wildlife pops ...
Quiz 2: Bio 160 Saunders
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is a function of the plasma membrane? A) control center of the cell B) protein synthesis C) fat synthesis D) intracellular digestion E) regulation of the passage of materi ...
... MULTIPLE CHOICE. Choose the one alternative that best completes the statement or answers the question. 1) Which of the following is a function of the plasma membrane? A) control center of the cell B) protein synthesis C) fat synthesis D) intracellular digestion E) regulation of the passage of materi ...
Lactic Acid Fermentation
... there is no oxygen available for yeast so the NADH builds up and NAD+ runs out. If NAD+ runs out, glycolysis itself will stop and there will be NO ATP made again. This will cause the organism to die. Therefore, a recycling program is needed to get the NADH back to NAD+. In alcohol fermentation, the ...
... there is no oxygen available for yeast so the NADH builds up and NAD+ runs out. If NAD+ runs out, glycolysis itself will stop and there will be NO ATP made again. This will cause the organism to die. Therefore, a recycling program is needed to get the NADH back to NAD+. In alcohol fermentation, the ...
BIO 330 Cell Biology Lecture Outline Spring 2011 Chapter 9
... Preparation for entry to Krebs cycle (citric acid cycle; tricarboxylic acid cycle) C. Fermentation In absence of oxygen Pyruvate is reduced by NADH to regenerate NAD+ Lactate fermentation Lactate dehydrogenase works in either direction depending on prevailing conditions in the cell Lactic acid produ ...
... Preparation for entry to Krebs cycle (citric acid cycle; tricarboxylic acid cycle) C. Fermentation In absence of oxygen Pyruvate is reduced by NADH to regenerate NAD+ Lactate fermentation Lactate dehydrogenase works in either direction depending on prevailing conditions in the cell Lactic acid produ ...
L10v02-glycolysis and TCA
... This is an overview of the relevant metabolic processes going on in the mitochondria. We have produced NADH Via glycolysis and citric acid cycle, which will be used for oxidative phosphorylation for a very much larger payoff in ATP ( on Wednesday). Here for the first time, you can see why we bre ...
... This is an overview of the relevant metabolic processes going on in the mitochondria. We have produced NADH Via glycolysis and citric acid cycle, which will be used for oxidative phosphorylation for a very much larger payoff in ATP ( on Wednesday). Here for the first time, you can see why we bre ...
Respiration
... Coenzyme NAD+ = electron acceptor NAD+ picks up 2e- and 2H+ NADH (stores E) NADH carries electrons to the electron transport ...
... Coenzyme NAD+ = electron acceptor NAD+ picks up 2e- and 2H+ NADH (stores E) NADH carries electrons to the electron transport ...
ch5_SP13x
... • Acidified ( high [H+] ) by action of the Electron Transport Chain (ETC) – H+ are pumped from matrix into this compartment – ATP synthase lets them back into the matrix ...
... • Acidified ( high [H+] ) by action of the Electron Transport Chain (ETC) – H+ are pumped from matrix into this compartment – ATP synthase lets them back into the matrix ...
Review Questions
... ____ 21. Where does the Calvin cycle take place? a. stroma of the chloroplast b. thylakoid membrane c. cytoplasm surrounding the chloroplast d. chlorophyll molecule e. outer membrane of the chloroplast ____ 22. When oxygen is released as a result of photosynthesis, it is a by-product of which of the ...
... ____ 21. Where does the Calvin cycle take place? a. stroma of the chloroplast b. thylakoid membrane c. cytoplasm surrounding the chloroplast d. chlorophyll molecule e. outer membrane of the chloroplast ____ 22. When oxygen is released as a result of photosynthesis, it is a by-product of which of the ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)