3.7 Energy-Rich Compounds
... generated during exergonic reactions and consumed in endergonic reactions. From the structure of ATP (Figure 3.12), it can be seen that only two of the phosphate bonds (ATP S ADP + Pi and ADP S AMP + Pi) are phosphoanhydrides and thus have free energies of hydrolysis greater than - 30 kJ. By contras ...
... generated during exergonic reactions and consumed in endergonic reactions. From the structure of ATP (Figure 3.12), it can be seen that only two of the phosphate bonds (ATP S ADP + Pi and ADP S AMP + Pi) are phosphoanhydrides and thus have free energies of hydrolysis greater than - 30 kJ. By contras ...
state university college at buffalo - Buffalo State College Faculty and
... 26. Phosphofructose Kinase (PFK) is an important regulatory enzyme in glycolysis. PFK is allosterically inhibited by ATP. Explain why this is considered an example of feedback inhibition. ...
... 26. Phosphofructose Kinase (PFK) is an important regulatory enzyme in glycolysis. PFK is allosterically inhibited by ATP. Explain why this is considered an example of feedback inhibition. ...
File
... Factors that affect the rate of photosynthesis: 1. Amount of sunlight 2. Amount of water 3. Amount of carbon dioxide CHEMOSYNTHESIS - Process by which some organisms produce energy through a chemical reaction. (ex. Bacteria) These are also autotrophs. CELLULAR RESPIRATION The process by which food ...
... Factors that affect the rate of photosynthesis: 1. Amount of sunlight 2. Amount of water 3. Amount of carbon dioxide CHEMOSYNTHESIS - Process by which some organisms produce energy through a chemical reaction. (ex. Bacteria) These are also autotrophs. CELLULAR RESPIRATION The process by which food ...
Chapter 6 Cellular Energy
... acetyl CoA, which enters the citric acid cycle Energy yield is 2 ATP, 6 NADH & 2 FADH2 Provides electrons for respiration ...
... acetyl CoA, which enters the citric acid cycle Energy yield is 2 ATP, 6 NADH & 2 FADH2 Provides electrons for respiration ...
Citric Acid (or Krebs) Cycle - BYU
... say exactly how many ATP we get. This is because some ATP is used to shuttle molecules in and out of the mitochondria and there is likely some “leaking” that occurs when protons from the intermembranous space accidentally escape by some other way than through the ATP synthase enzyme complex. However ...
... say exactly how many ATP we get. This is because some ATP is used to shuttle molecules in and out of the mitochondria and there is likely some “leaking” that occurs when protons from the intermembranous space accidentally escape by some other way than through the ATP synthase enzyme complex. However ...
Chapter 8- An Introduction to Microbial Metabolism
... molecules of ATP for every 1 molecule of glucose. The only way that glycolysis can run is for NAD+ to pick up electrons and a proton (see glycolysis summary equation). There must be a way to oxidize NADH back to NAD+. In aerobic organisms the NAD+ is regenerated when NADH delivers the H+ and electro ...
... molecules of ATP for every 1 molecule of glucose. The only way that glycolysis can run is for NAD+ to pick up electrons and a proton (see glycolysis summary equation). There must be a way to oxidize NADH back to NAD+. In aerobic organisms the NAD+ is regenerated when NADH delivers the H+ and electro ...
Producer
... Energy Flow • Energy flows through an ecosystem in one direction from the sun or inorganic molecules to producers (autotrophs) and then to consumers ...
... Energy Flow • Energy flows through an ecosystem in one direction from the sun or inorganic molecules to producers (autotrophs) and then to consumers ...
Interactions of Life
... May not be enough resources to support all members of the population. Its likely that members will begin to die from lack of resources such as food and water. ...
... May not be enough resources to support all members of the population. Its likely that members will begin to die from lack of resources such as food and water. ...
0-bacterial-physiology&growth
... •The final electron acceptor is an organic molecule such as nitrate, sulfate or CO2 •There is also: - Glycolysis, - Krebs cycle - Oxidative phosphorylation •Because nitrate, sulfate or CO2 are not good electron acceptor as oxygen, the net yield of ATP molecules is less than it is with aerobic respir ...
... •The final electron acceptor is an organic molecule such as nitrate, sulfate or CO2 •There is also: - Glycolysis, - Krebs cycle - Oxidative phosphorylation •Because nitrate, sulfate or CO2 are not good electron acceptor as oxygen, the net yield of ATP molecules is less than it is with aerobic respir ...
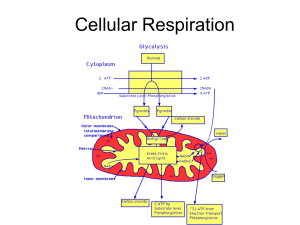
Cellular Respiration
... 7. The electrons from step 2 enter step 3 and make how many ATP? 8. From splitting 1 glucose how many total ATP are produced in cell respiration? 9. Anaerobic respiration is a type of cell respiration that requires no oxygen and only produces ___ ATP. 10. Alcoholic fermentation is used in ___ and la ...
... 7. The electrons from step 2 enter step 3 and make how many ATP? 8. From splitting 1 glucose how many total ATP are produced in cell respiration? 9. Anaerobic respiration is a type of cell respiration that requires no oxygen and only produces ___ ATP. 10. Alcoholic fermentation is used in ___ and la ...
SR 50(4) 42-43 (Test Your Knowledge)
... a) They have a plasma membrane b) They have DNA c) They divide by binary fission d) They have nuclei ...
... a) They have a plasma membrane b) They have DNA c) They divide by binary fission d) They have nuclei ...
2.3 Carbon Compounds
... that contain bonds between carbon atoms, while inorganic chemistry is the study of all other compounds In the early 1800s, many chemists called the compounds created by organisms “organic,” believing they were fundamentally different from compounds in nonliving things. ...
... that contain bonds between carbon atoms, while inorganic chemistry is the study of all other compounds In the early 1800s, many chemists called the compounds created by organisms “organic,” believing they were fundamentally different from compounds in nonliving things. ...
Bio150 Chapter 7
... – Many prokaryotes use sulfur, nitrate, carbon dioxide or even inorganic metals ...
... – Many prokaryotes use sulfur, nitrate, carbon dioxide or even inorganic metals ...
2.3_Carbon_Compounds
... that contain bonds between carbon atoms, while inorganic chemistry is the study of all other compounds In the early 1800s, many chemists called the compounds created by organisms “organic,” believing they were fundamentally different from compounds in nonliving things. ...
... that contain bonds between carbon atoms, while inorganic chemistry is the study of all other compounds In the early 1800s, many chemists called the compounds created by organisms “organic,” believing they were fundamentally different from compounds in nonliving things. ...
Lecture Presentation to accompany Principles of Life
... • 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism • 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy • 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy ...
... • 6.1 ATP, Reduced Coenzymes, and Chemiosmosis Play Important Roles in Biological Energy Metabolism • 6.2 Carbohydrate Catabolism in the Presence of Oxygen Releases a Large Amount of Energy • 6.3 Carbohydrate Catabolism in the Absence of Oxygen Releases a Small Amount of Energy ...
4 Metabolism and Enzymes
... electron transport chain is molecular oxygen (O2) in aerobes. • Anaerobic respiration: The final electron acceptor in the electron transport chain is not O2. Yields less energy than aerobic respiration because only part of the Krebs cycles operations under anaerobic conditions. Obligate anaerobes pe ...
... electron transport chain is molecular oxygen (O2) in aerobes. • Anaerobic respiration: The final electron acceptor in the electron transport chain is not O2. Yields less energy than aerobic respiration because only part of the Krebs cycles operations under anaerobic conditions. Obligate anaerobes pe ...
energy
... When a consumer eats a plant/animal for food -both Energy and matter are passed to the consumer ...
... When a consumer eats a plant/animal for food -both Energy and matter are passed to the consumer ...
Chapter 6, Section 3
... Organic: contains carbon ◦ All living things contain carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P) Monomer: created when C,H,O, N, P bond together to form small molecules Polymer: large compounds that are formed by joining monomers together ...
... Organic: contains carbon ◦ All living things contain carbon (C), hydrogen (H), oxygen (O), nitrogen (N), and phosphorus (P) Monomer: created when C,H,O, N, P bond together to form small molecules Polymer: large compounds that are formed by joining monomers together ...
Chapter 9: How do cells harvest energy?
... Chapter 9: Cellular Respiration-Harvesting Chemical Energy I. ...
... Chapter 9: Cellular Respiration-Harvesting Chemical Energy I. ...
Which of the following is a coenzyme associated with cellular
... When NADH produced during the transition reaction and Krebs cycle delivers electrons to the electron transport system, _____ ATP is/are produced. ...
... When NADH produced during the transition reaction and Krebs cycle delivers electrons to the electron transport system, _____ ATP is/are produced. ...
A chemist has discovered a drug that blocks
... c. human cells must also perform glycolysis; the drug might also poison them d. this step in the pathway of glycolysis can be skipped in bacteria, but not in humans e. glycolysis can occur without the action of enzymes 3. How do you account for a situation in which a person can utilize only fatty ac ...
... c. human cells must also perform glycolysis; the drug might also poison them d. this step in the pathway of glycolysis can be skipped in bacteria, but not in humans e. glycolysis can occur without the action of enzymes 3. How do you account for a situation in which a person can utilize only fatty ac ...
Ch. 9 - Ltcconline.net
... d. this process stores the energy that the ATP synthase will use to make ATP e. ATP synthase uses chemiosmosis of H+ ions diffusing down concentration gradient to attach P to ADP B. Glycolysis harvests chemical energy by oxidizing glucose into pyruvic acid 1. Glycolysis takes 10 chemical steps to tu ...
... d. this process stores the energy that the ATP synthase will use to make ATP e. ATP synthase uses chemiosmosis of H+ ions diffusing down concentration gradient to attach P to ADP B. Glycolysis harvests chemical energy by oxidizing glucose into pyruvic acid 1. Glycolysis takes 10 chemical steps to tu ...
ecosystem - Wando High School
... molecules such as carbohydrates, proteins, lipids, and nucleic acids. For example, when consumers eat plants and/or animals, some of the compounds are used for energy; others are converted to compounds that are incorporated into the consumer’s body. Still other compounds such as methane and other ga ...
... molecules such as carbohydrates, proteins, lipids, and nucleic acids. For example, when consumers eat plants and/or animals, some of the compounds are used for energy; others are converted to compounds that are incorporated into the consumer’s body. Still other compounds such as methane and other ga ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)