Cell Physiology
... – to further oxidize NADH and FADH2 and transfer their energy to ATP – to regenerate NAD+ and FAD and make them available again to earlier reaction steps ...
... – to further oxidize NADH and FADH2 and transfer their energy to ATP – to regenerate NAD+ and FAD and make them available again to earlier reaction steps ...
answer key
... form of ATP? What is the total potential number of moles of ATP available from the combustion of glucose if the process were 100% efficient? (VV p. 442 Problem 6) Efficiency = 38(-48.1 kJ mol-1/-2823.2 kJ.mol-1) x 100 = 65% (5 pts) Total potential ATP = -2823.2 kJ mol-1/-48.1 kJ.mol-1 = 58 (5 pts) ...
... form of ATP? What is the total potential number of moles of ATP available from the combustion of glucose if the process were 100% efficient? (VV p. 442 Problem 6) Efficiency = 38(-48.1 kJ mol-1/-2823.2 kJ.mol-1) x 100 = 65% (5 pts) Total potential ATP = -2823.2 kJ mol-1/-48.1 kJ.mol-1 = 58 (5 pts) ...
Chapter 3 Notes
... As abiotic factors change, the environment also changes As well, as one population within the ecosystem changes, those populations that interact with them will also change Populations are also able to change their environment over time, particularly after a major change to that environment ...
... As abiotic factors change, the environment also changes As well, as one population within the ecosystem changes, those populations that interact with them will also change Populations are also able to change their environment over time, particularly after a major change to that environment ...
PowerPoint
... as genetic material to code for proteins It would have had ribosomes to produce proteins It would have been surrounded by a phospholipid bilayer It would have used glycolysis as a means of extracting energy from glucose ...
... as genetic material to code for proteins It would have had ribosomes to produce proteins It would have been surrounded by a phospholipid bilayer It would have used glycolysis as a means of extracting energy from glucose ...
Derived copy of Bis2A 07.2 Fermentation
... Visit this site2 to see anaerobic cellular respiration in action. Other fermentation methods occur in bacteria. Many bacteria are facultatively aerobes. This means that they can switch between aerobic and anaerobic growth depending on the availability of oxygen. Certain bacteria, like Clostridia bac ...
... Visit this site2 to see anaerobic cellular respiration in action. Other fermentation methods occur in bacteria. Many bacteria are facultatively aerobes. This means that they can switch between aerobic and anaerobic growth depending on the availability of oxygen. Certain bacteria, like Clostridia bac ...
Cellular Respiration Powerpoint
... • This cycle goes around twice for each molecule of glucose • And for every turn of the cycle, 1 ATP is produced • In total, 2 ATP are produced from the Citric Acid/Kreb’s Cycle (because the cycle rotates twice) ...
... • This cycle goes around twice for each molecule of glucose • And for every turn of the cycle, 1 ATP is produced • In total, 2 ATP are produced from the Citric Acid/Kreb’s Cycle (because the cycle rotates twice) ...
Energy Pathways and Anaerobic Metabolism
... your body comes to a fork in the road… Glycolysis 2 Pyruvate O2 absent ...
... your body comes to a fork in the road… Glycolysis 2 Pyruvate O2 absent ...
Mock Exam 2 BY 123 - Cusic Supplemental Instruction
... 32. _____________ is used in to create ATP by the high H+ concentration in the __________ and the low H+ concentration in _____________ in animal cells. ____________ Is used in to create ATP by the high H+ concentration in the __________ and the low H+ concentration in _____________ in plant cells. ...
... 32. _____________ is used in to create ATP by the high H+ concentration in the __________ and the low H+ concentration in _____________ in animal cells. ____________ Is used in to create ATP by the high H+ concentration in the __________ and the low H+ concentration in _____________ in plant cells. ...
Campbell`s Biology, 9e (Reece et al.)
... A) The more electronegative atom is reduced, and energy is released. B) The more electronegative atom is reduced, and energy is consumed. C) The more electronegative atom is oxidized, and energy is consumed. D) The more electronegative atom is oxidized, and energy is released. E) The more electroneg ...
... A) The more electronegative atom is reduced, and energy is released. B) The more electronegative atom is reduced, and energy is consumed. C) The more electronegative atom is oxidized, and energy is consumed. D) The more electronegative atom is oxidized, and energy is released. E) The more electroneg ...
RuBisCO and C4 plants
... dioxide and water to give two glycerate-3-phosphate (GP) molecules, which can be utilised in the ‘C3’ Calvin cycle. But this reaction is very slow at low carbon dioxide concentrations. Rubisco also catalyses another reaction – the oxygenation of RuBP. When RuBP reacts with oxygen, it gives a molecul ...
... dioxide and water to give two glycerate-3-phosphate (GP) molecules, which can be utilised in the ‘C3’ Calvin cycle. But this reaction is very slow at low carbon dioxide concentrations. Rubisco also catalyses another reaction – the oxygenation of RuBP. When RuBP reacts with oxygen, it gives a molecul ...
Understanding the origin and organization of
... Oily membranes in a water medium are proton semiconductors http://www.chemistry.wustl.edu/~edudev/LabTutorials/Cytochromes/cytochromes.html ...
... Oily membranes in a water medium are proton semiconductors http://www.chemistry.wustl.edu/~edudev/LabTutorials/Cytochromes/cytochromes.html ...
Cell Respiration - Glycolysis PPT
... 2.F.1 Glycolysis rearranges the bonds in glucose molecules, releasing free energy to form ATP from ADP and inorganic phosphate, and resulting in the production of pyruvate. ...
... 2.F.1 Glycolysis rearranges the bonds in glucose molecules, releasing free energy to form ATP from ADP and inorganic phosphate, and resulting in the production of pyruvate. ...
a ANSWER - Cornerstone Charter Academy
... 1. Describe microscopes and their importance in viewing cellular structure 2. Distinguish between prokaryotic and eukaryotic cells 3. Describe the structure of cell membranes and how membrane structure relates to function 4. Discuss ways that cellular organelles are involved in the manufacture and ...
... 1. Describe microscopes and their importance in viewing cellular structure 2. Distinguish between prokaryotic and eukaryotic cells 3. Describe the structure of cell membranes and how membrane structure relates to function 4. Discuss ways that cellular organelles are involved in the manufacture and ...
No Slide Title - Suffolk County Community College
... phosphorylation: H2S, S, NH3, NO2-, H2, Fe2+, CO (electron acceptor in respiration) - e.g. Few bacteria, e.g. Pseudomonas Chemoheterotrophs - electrons from H in organics for energy (redox reactions) - C from same organics for carbon (respiration) - compounds used for oxidative phosphorylation: O2, ...
... phosphorylation: H2S, S, NH3, NO2-, H2, Fe2+, CO (electron acceptor in respiration) - e.g. Few bacteria, e.g. Pseudomonas Chemoheterotrophs - electrons from H in organics for energy (redox reactions) - C from same organics for carbon (respiration) - compounds used for oxidative phosphorylation: O2, ...
Organic Molecules
... All four have in common… • Carbon backbone • Other atoms, usually H,O,N,P,and/or S • These atoms form functional groups, which we can recognize • These groups replace the H that would be in a typical hydrocarbon ...
... All four have in common… • Carbon backbone • Other atoms, usually H,O,N,P,and/or S • These atoms form functional groups, which we can recognize • These groups replace the H that would be in a typical hydrocarbon ...
Enzymes & Energy
... transferred to ADP, phosphorylating it to ATP. 2 more ATPs are made. Two pyruvic acid molecules are formed from the single original glucose. ...
... transferred to ADP, phosphorylating it to ATP. 2 more ATPs are made. Two pyruvic acid molecules are formed from the single original glucose. ...
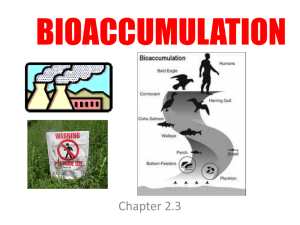
Bioaccumulation
... • PCBs concentrate in the blubber of the whale • When the blubber is burned for energy, the PCBs are released into bloodstream (where they affect immune function). • Calves are born with the same PCB level as mother and then obtain more through milk. • PCB’s will affect the reproductive cycles of or ...
... • PCBs concentrate in the blubber of the whale • When the blubber is burned for energy, the PCBs are released into bloodstream (where they affect immune function). • Calves are born with the same PCB level as mother and then obtain more through milk. • PCB’s will affect the reproductive cycles of or ...
Cellular respiration
... 3. Describe the conditions under which an athlete would be relying mostly on the Aerobic System to produced ATP. 4. What must proteins and fats be converted into in order to be used as fuel for the Aerobic System? ...
... 3. Describe the conditions under which an athlete would be relying mostly on the Aerobic System to produced ATP. 4. What must proteins and fats be converted into in order to be used as fuel for the Aerobic System? ...
The electron transport chain is a part of cellular respiration. The
... This answer suggests the student may understand that photolysis occurs during the lightdependent reactions in photosynthesis, but does not understand that oxygen is not split and combined with carbon to form carbon dioxide during photosynthesis, and that the plant is undergoing cellular respiration, ...
... This answer suggests the student may understand that photolysis occurs during the lightdependent reactions in photosynthesis, but does not understand that oxygen is not split and combined with carbon to form carbon dioxide during photosynthesis, and that the plant is undergoing cellular respiration, ...
A Practice Reactions Quiz -
... DIRECTIONS A) Write complete balanced equations for the following reactions. B) Label each reaction as either SYN, DEC, SR, DR, or COMB. C) Place a star next to any reaction which required knowledge of oxidation numbers. D) Finally, find the two reactions below which do not actually take place. Writ ...
... DIRECTIONS A) Write complete balanced equations for the following reactions. B) Label each reaction as either SYN, DEC, SR, DR, or COMB. C) Place a star next to any reaction which required knowledge of oxidation numbers. D) Finally, find the two reactions below which do not actually take place. Writ ...
Lesson 3 Where do metals come from
... Where do metals come from? Baseline (Flightpath D): Define oxidation and reduction in terms of oxygen. Describe how metals can be extracted. Further (Flightpath C&B) Identify species that are being oxidised and reduced in a chemical reaction. Explain why some metals are found uncombined in the Earth ...
... Where do metals come from? Baseline (Flightpath D): Define oxidation and reduction in terms of oxygen. Describe how metals can be extracted. Further (Flightpath C&B) Identify species that are being oxidised and reduced in a chemical reaction. Explain why some metals are found uncombined in the Earth ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)