Syntrophic linkage between predatory Carpediemonas and
... proceeds via glycolysis followed by the decarboxylation of pyruvate (Müller et al., 2012). In strictly anerobic microbial eukaryotes, pyruvate decarboxylation often takes place in mitochondria that lost their capability to respire oxygen (Boxma et al., 2005). These mitochondria recycle reducing equi ...
... proceeds via glycolysis followed by the decarboxylation of pyruvate (Müller et al., 2012). In strictly anerobic microbial eukaryotes, pyruvate decarboxylation often takes place in mitochondria that lost their capability to respire oxygen (Boxma et al., 2005). These mitochondria recycle reducing equi ...
Chapter 6 How Cells Harvest Chemical Energy In eukaryotes, cellular respiration
... 6.9 The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH2 molecules The citric acid cycle – is also called the Krebs cycle (after the German-British researcher Hans Krebs, who worked out much of this pathway in the 1930s), – completes the oxidation of ...
... 6.9 The citric acid cycle completes the oxidation of organic molecules, generating many NADH and FADH2 molecules The citric acid cycle – is also called the Krebs cycle (after the German-British researcher Hans Krebs, who worked out much of this pathway in the 1930s), – completes the oxidation of ...
Metabolism
... 1. Pyruvic acid from glycolysis is converted to acetyl coenzyme A (acetyl CoA). 2. Acetyl CoA enters the Krebs cycle and forms 2 ATP, carbon dioxide, and hydrogen. 3. Hydrogen in the cell combines with two coenzymes that carry it to the electron transport chain. 4. Electron transport chain recombine ...
... 1. Pyruvic acid from glycolysis is converted to acetyl coenzyme A (acetyl CoA). 2. Acetyl CoA enters the Krebs cycle and forms 2 ATP, carbon dioxide, and hydrogen. 3. Hydrogen in the cell combines with two coenzymes that carry it to the electron transport chain. 4. Electron transport chain recombine ...
Learning Objectives
... 1. Pyruvic acid from glycolysis is converted to acetyl coenzyme A (acetyl CoA). 2. Acetyl CoA enters the Krebs cycle and forms 2 ATP, carbon dioxide, and hydrogen. 3. Hydrogen in the cell combines with two coenzymes that carry it to the electron transport chain. 4. Electron transport chain recombine ...
... 1. Pyruvic acid from glycolysis is converted to acetyl coenzyme A (acetyl CoA). 2. Acetyl CoA enters the Krebs cycle and forms 2 ATP, carbon dioxide, and hydrogen. 3. Hydrogen in the cell combines with two coenzymes that carry it to the electron transport chain. 4. Electron transport chain recombine ...
Dormancy of cells and organisms -strategies for survival and
... • Reveal strategies employed by different organisms that facilitate their long term survival in a dormant form • Widening the basis of our knowledge on the molecular details of strategies employed by 5 model organisms • Resolve whether these strategies can be used to develop preservation (“ reversib ...
... • Reveal strategies employed by different organisms that facilitate their long term survival in a dormant form • Widening the basis of our knowledge on the molecular details of strategies employed by 5 model organisms • Resolve whether these strategies can be used to develop preservation (“ reversib ...
Lecture_12
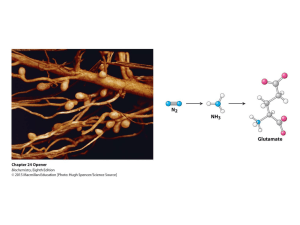
... Although the atmosphere is approximately 80% nitrogen, this vital element is not available to the majority of organisms. However, a few organisms, such as the diazotrophic (nitrogen-fixing) bacteria, can convert nitrogen gas (N2) into the biochemically more useful NH3. ...
... Although the atmosphere is approximately 80% nitrogen, this vital element is not available to the majority of organisms. However, a few organisms, such as the diazotrophic (nitrogen-fixing) bacteria, can convert nitrogen gas (N2) into the biochemically more useful NH3. ...
Exam 1 with Key
... At pH 7.65, log[H+] = -7.65, so [H+] = 10-7.65 = 2.24x10-8 M At pH 6.87, log[H+] = -6.87, so [H+] = 10-6.87 = 1.35x10-7 M The difference in [H+] is 1.35x10-7 M - 2.24x10-8 M = 1.13x10-7 M. Since the reaction is quantitative, the same amount of acetylcholine is initially present. (0.015L)(1.13x10-7 M ...
... At pH 7.65, log[H+] = -7.65, so [H+] = 10-7.65 = 2.24x10-8 M At pH 6.87, log[H+] = -6.87, so [H+] = 10-6.87 = 1.35x10-7 M The difference in [H+] is 1.35x10-7 M - 2.24x10-8 M = 1.13x10-7 M. Since the reaction is quantitative, the same amount of acetylcholine is initially present. (0.015L)(1.13x10-7 M ...
Lecture 13: Krebs` Cycle / Citric Acid
... Phosphogluconate pathway / Warburg and Dicken’s pathway The pentose phosphate pathway occurs in the cytoplasm outside the mitochondria and it is an alternative pathway to glycolysis and Kreb’s cycle. The presence of some compounds like iodoacetate, fluorides, arsenates etc. inhibit some steps in gly ...
... Phosphogluconate pathway / Warburg and Dicken’s pathway The pentose phosphate pathway occurs in the cytoplasm outside the mitochondria and it is an alternative pathway to glycolysis and Kreb’s cycle. The presence of some compounds like iodoacetate, fluorides, arsenates etc. inhibit some steps in gly ...
2t.7 Cellular work
... Some phosphorylated enzyme substrates are activated for subsequent reactions they would not ordinarily undergo. The process of activation often involves a coupled reaction-an energeticallyunfauorable reaction is made to occur by being linked to a reaction that is energetically ueryfauorable (uery ex ...
... Some phosphorylated enzyme substrates are activated for subsequent reactions they would not ordinarily undergo. The process of activation often involves a coupled reaction-an energeticallyunfauorable reaction is made to occur by being linked to a reaction that is energetically ueryfauorable (uery ex ...
THE CITRIC ACID CYCLE
... • Compare to ATP phosphate hydrolysis at -30 kJ/mole • We preserve that energy by making GTP • This reaction utilizes a swinging histidine side chain to transfer the PO42- group from succinyl phosphate to ...
... • Compare to ATP phosphate hydrolysis at -30 kJ/mole • We preserve that energy by making GTP • This reaction utilizes a swinging histidine side chain to transfer the PO42- group from succinyl phosphate to ...
... expression (phenotypic changes) in the bacteria. An example is that mucins induce an up-regulation of degradative enzymes within bacteria that interact with them. The bacteria use these enzymes to degrade the mucins and then use the degradative products as nutrients. Not all bacteria express these e ...
A2 level Biology Revision Notes - A
... Imagine situation in which single algal cell, capable asexual reproduction, is placed in a newly created pond: Summer & so plenty light & temp of water around 12oC, mineral nutrients being added to water, In these circumstances algal cell divides rapidly as all factors needed for growth of populatio ...
... Imagine situation in which single algal cell, capable asexual reproduction, is placed in a newly created pond: Summer & so plenty light & temp of water around 12oC, mineral nutrients being added to water, In these circumstances algal cell divides rapidly as all factors needed for growth of populatio ...
Presentation
... ATP synthesis can be uncoupled: if a different H+ diffusion channel is inserted into the mitochondrial membrane, the energy of the diffusion is lost as heat. The protein thermogenin occurs in human ...
... ATP synthesis can be uncoupled: if a different H+ diffusion channel is inserted into the mitochondrial membrane, the energy of the diffusion is lost as heat. The protein thermogenin occurs in human ...
COURSE NTRODUCTION
... affect the growth of bacteria. Nutrients, temperature, water activity, pH, chemical inhibitors, and atmosphere can all be used to control bacterial growth. Bacteria, like any living organism, require food and water to carry on their life processes. Nutrients must be in solution before they can be tr ...
... affect the growth of bacteria. Nutrients, temperature, water activity, pH, chemical inhibitors, and atmosphere can all be used to control bacterial growth. Bacteria, like any living organism, require food and water to carry on their life processes. Nutrients must be in solution before they can be tr ...
oxidation - mustafaaltinisik.org.uk
... 1. Pyruvic acid from glycolysis is converted to acetyl coenzyme A (acetyl CoA). 2. Acetyl CoA enters the Krebs cycle and forms 2 ATP, carbon dioxide, and hydrogen. 3. Hydrogen in the cell combines with two coenzymes that carry it to the electron transport chain. 4. Electron transport chain recombine ...
... 1. Pyruvic acid from glycolysis is converted to acetyl coenzyme A (acetyl CoA). 2. Acetyl CoA enters the Krebs cycle and forms 2 ATP, carbon dioxide, and hydrogen. 3. Hydrogen in the cell combines with two coenzymes that carry it to the electron transport chain. 4. Electron transport chain recombine ...
Chapter 5
... chemical reaction between an electron donor (such as NADH) and an electron acceptor (such as O2) to the transfer of H+ ions across a membrane, through a set of mediating biochemical reactions. ...
... chemical reaction between an electron donor (such as NADH) and an electron acceptor (such as O2) to the transfer of H+ ions across a membrane, through a set of mediating biochemical reactions. ...
A Mad Scientist`s Chemistry Presentation
... “changes to” or “forms.” • The arrow may point in one direction or in both directions. • An arrow that points in both directions indicates that the products can react with each other to re-form the reactants. ...
... “changes to” or “forms.” • The arrow may point in one direction or in both directions. • An arrow that points in both directions indicates that the products can react with each other to re-form the reactants. ...
Cellular Respiration
... from glucose to oxygen involves many steps. • The first step is an electron acceptor called NAD+. – NAD is made by cells from niacin, a B vitamin. – The transfer of electrons from organic fuel to NAD+ reduces it to NADH. ...
... from glucose to oxygen involves many steps. • The first step is an electron acceptor called NAD+. – NAD is made by cells from niacin, a B vitamin. – The transfer of electrons from organic fuel to NAD+ reduces it to NADH. ...
Organic Chemistry – Review #2 Vocabulary Adhesion Cohesion
... o Valence shell enables easy formation of four covalent bonds o Covalent bonds involve _____________ of ________________ between two atoms ...
... o Valence shell enables easy formation of four covalent bonds o Covalent bonds involve _____________ of ________________ between two atoms ...
Chapter 9 - Cellular Respiration
... 3. Acetate combines with coenzyme A to form the very reactive molecule acetyl CoA. Acetyl CoA is now ready to feed its acetyl group into the citric acid cycle for further oxidation. The citric acid cycle is also called the Krebs cycle in honor of Hans Krebs, who was largely responsible for eluci ...
... 3. Acetate combines with coenzyme A to form the very reactive molecule acetyl CoA. Acetyl CoA is now ready to feed its acetyl group into the citric acid cycle for further oxidation. The citric acid cycle is also called the Krebs cycle in honor of Hans Krebs, who was largely responsible for eluci ...
Alcohols Oxidation by oxygen O2 in presence of
... , and hetero poly acid are necessary for this reaction. In this condition the benzylic alcohols type 1 and type 2 with different substitutions on the phenyl ring with suitable yield were oxidized to ketons and aldehydes without more oxidation into carboxylic acids. Key words: heteropolyacid, catalys ...
... , and hetero poly acid are necessary for this reaction. In this condition the benzylic alcohols type 1 and type 2 with different substitutions on the phenyl ring with suitable yield were oxidized to ketons and aldehydes without more oxidation into carboxylic acids. Key words: heteropolyacid, catalys ...
Biol 1406 notes Ch 9 8thed
... The output from this step is the only ATP generated directly by the citric acid cycle. Most of the ATP produced by respiration results from oxidative phosphorylation, as the NADH and FADH2 produced by the citric acid cycle relay the electrons extracted from food to the electron transport chain. This ...
... The output from this step is the only ATP generated directly by the citric acid cycle. Most of the ATP produced by respiration results from oxidative phosphorylation, as the NADH and FADH2 produced by the citric acid cycle relay the electrons extracted from food to the electron transport chain. This ...
Microbial metabolism
Microbial metabolism is the means by which a microbe obtains the energy and nutrients (e.g. carbon) it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe’s ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.== Types of microbial metabolism ==All microbial metabolisms can be arranged according to three principles:1. How the organism obtains carbon for synthesising cell mass: autotrophic – carbon is obtained from carbon dioxide (CO2) heterotrophic – carbon is obtained from organic compounds mixotrophic – carbon is obtained from both organic compounds and by fixing carbon dioxide2. How the organism obtains reducing equivalents used either in energy conservation or in biosynthetic reactions: lithotrophic – reducing equivalents are obtained from inorganic compounds organotrophic – reducing equivalents are obtained from organic compounds3. How the organism obtains energy for living and growing: chemotrophic – energy is obtained from external chemical compounds phototrophic – energy is obtained from lightIn practice, these terms are almost freely combined. Typical examples are as follows: chemolithoautotrophs obtain energy from the oxidation of inorganic compounds and carbon from the fixation of carbon dioxide. Examples: Nitrifying bacteria, Sulfur-oxidizing bacteria, Iron-oxidizing bacteria, Knallgas-bacteria photolithoautotrophs obtain energy from light and carbon from the fixation of carbon dioxide, using reducing equivalents from inorganic compounds. Examples: Cyanobacteria (water (H2O) as reducing equivalent donor), Chlorobiaceae, Chromatiaceae (hydrogen sulfide (H2S) as reducing equivalent donor), Chloroflexus (hydrogen (H2) as reducing equivalent donor) chemolithoheterotrophs obtain energy from the oxidation of inorganic compounds, but cannot fix carbon dioxide (CO2). Examples: some Thiobacilus, some Beggiatoa, some Nitrobacter spp., Wolinella (with H2 as reducing equivalent donor), some Knallgas-bacteria, some sulfate-reducing bacteria chemoorganoheterotrophs obtain energy, carbon, and reducing equivalents for biosynthetic reactions from organic compounds. Examples: most bacteria, e. g. Escherichia coli, Bacillus spp., Actinobacteria photoorganoheterotrophs obtain energy from light, carbon and reducing equivalents for biosynthetic reactions from organic compounds. Some species are strictly heterotrophic, many others can also fix carbon dioxide and are mixotrophic. Examples: Rhodobacter, Rhodopseudomonas, Rhodospirillum, Rhodomicrobium, Rhodocyclus, Heliobacterium, Chloroflexus (alternatively to photolithoautotrophy with hydrogen)