Amino acid catabolism I
... 3. ammonia production in the large intestine by bacteria portal vein, direct transport of ammonia. Urea cycle Function: 1. prevents ammonia levels from rising too high when large amounts of amino acids are catabolized 2. urea cycle enzymes: extrahepatic arginine synthesis ...
... 3. ammonia production in the large intestine by bacteria portal vein, direct transport of ammonia. Urea cycle Function: 1. prevents ammonia levels from rising too high when large amounts of amino acids are catabolized 2. urea cycle enzymes: extrahepatic arginine synthesis ...
Metabolism Power Point
... molecules so increase rate of a reaction BUT. . Above a certain temperature, activity begins to decline because the enzyme begins to denature. So rate of chemical reaction increases with temperature up to optimum, then decreases ...
... molecules so increase rate of a reaction BUT. . Above a certain temperature, activity begins to decline because the enzyme begins to denature. So rate of chemical reaction increases with temperature up to optimum, then decreases ...
10) water soluble vitamins
... Food Sources • Preformed niacin from meat, poultry fish and enriched or whole grain products • Beef and processed meats are substantial contributors in U.S. diet ...
... Food Sources • Preformed niacin from meat, poultry fish and enriched or whole grain products • Beef and processed meats are substantial contributors in U.S. diet ...
Unit 4: Cellular Energy Study Guide
... By the time the electrons from photosystem II reach photosystem I, they have very little energy. A second light source must re-charge these electrons when they reach photosystem I. The pigment that absorbs light in photosystem I is called P700 because it absorbs light at 700 nanometers. The electron ...
... By the time the electrons from photosystem II reach photosystem I, they have very little energy. A second light source must re-charge these electrons when they reach photosystem I. The pigment that absorbs light in photosystem I is called P700 because it absorbs light at 700 nanometers. The electron ...
Cancer Cachexia: an update on the aetiology and management
... coupling from oxidative phosphorylation is released as heat energy instead of ATP ...
... coupling from oxidative phosphorylation is released as heat energy instead of ATP ...
Chapter 17 – Amino Acid Metabolism
... 2) two re-dox centers, one of which is a nitrogenase Composed of iron and molybdenum that reduces N2 to NH4 ...
... 2) two re-dox centers, one of which is a nitrogenase Composed of iron and molybdenum that reduces N2 to NH4 ...
Chapter 8 Notes
... Substrate Specificity of Enzymes • The reactant that an enzyme acts on is called the enzyme’s substrate • The enzyme binds to its substrate, forming an enzyme-substrate complex • The active site is the region on the enzyme where the substrate binds • Induced fit of a substrate brings chemical group ...
... Substrate Specificity of Enzymes • The reactant that an enzyme acts on is called the enzyme’s substrate • The enzyme binds to its substrate, forming an enzyme-substrate complex • The active site is the region on the enzyme where the substrate binds • Induced fit of a substrate brings chemical group ...
Name - Phillips Scientific Methods
... 5. How many CO2 molecules are produced per pyruvate? _____ per glucose? _____ 6. How many NADH2 molecules are produced per pyruvate? ______ 7. How many FADH2 molecules are produced per glucose? _______ 8. How many ATP are produced per glucose molecule in the Kreb’s cycle? _______ ...
... 5. How many CO2 molecules are produced per pyruvate? _____ per glucose? _____ 6. How many NADH2 molecules are produced per pyruvate? ______ 7. How many FADH2 molecules are produced per glucose? _______ 8. How many ATP are produced per glucose molecule in the Kreb’s cycle? _______ ...
Ch20.2 Amino-acids-degradation and synthesis
... CO2, the dehydrated form of carbonic acid, is carried by the vitamin biotin, which is a prosthetic group for most carboxylation reactions, but is not considered a member of the ...
... CO2, the dehydrated form of carbonic acid, is carried by the vitamin biotin, which is a prosthetic group for most carboxylation reactions, but is not considered a member of the ...
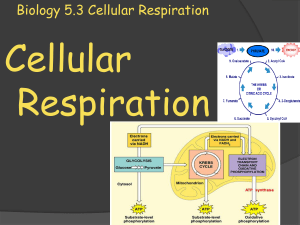
Biology 5.3 Cellular Respiration
... pyruvate during glycolysis. Glycolysis is an anaerobic process (no oxygen required), and it results in a gain of two ATP molecules. In the second stage of cellular respiration, the pyruvate passes through either aerobic respiration (requires oxygen) or fermentation. When oxygen is not present, ferme ...
... pyruvate during glycolysis. Glycolysis is an anaerobic process (no oxygen required), and it results in a gain of two ATP molecules. In the second stage of cellular respiration, the pyruvate passes through either aerobic respiration (requires oxygen) or fermentation. When oxygen is not present, ferme ...
Kate Buckman Modified session plan: Fermentation: one part in a
... However in the absence of oxygen this pathway is not an option. As NADH is only present in small amounts, it must be oxidized back to NAD+ in order for ATP production to continue through glycolysis. Anaerobic organisms (like yeast) or cells functioning under insufficient oxygen conditions (such as h ...
... However in the absence of oxygen this pathway is not an option. As NADH is only present in small amounts, it must be oxidized back to NAD+ in order for ATP production to continue through glycolysis. Anaerobic organisms (like yeast) or cells functioning under insufficient oxygen conditions (such as h ...
Ethylene Glycol Poisoning
... Occur with the ingestion of at least 100 cc of ethylene glycol Occur 6-18 days after the ingestion of ethylene glycol Postmortem studies attribute this to inflammation around the nerve from oxalate microcrystal deposition ...
... Occur with the ingestion of at least 100 cc of ethylene glycol Occur 6-18 days after the ingestion of ethylene glycol Postmortem studies attribute this to inflammation around the nerve from oxalate microcrystal deposition ...
Name - Northern Highlands
... b. Glycolysis produces so little ATP that the drug will have little effect. c. Human cells also perform glycolysis; the drug might also poison them. d. Bacteria do not perform glycolysis. 11. A glucose molecule is completely oxidized in glycolysis and the citric acid cycle, but these two processes y ...
... b. Glycolysis produces so little ATP that the drug will have little effect. c. Human cells also perform glycolysis; the drug might also poison them. d. Bacteria do not perform glycolysis. 11. A glucose molecule is completely oxidized in glycolysis and the citric acid cycle, but these two processes y ...
QUIZ #4 LIPID STRUCTURES AND METABOLISM
... d. Most of our cholesterol is converted into bile salts and acids e. The carbons in cholesterol can be traced to carbohydrates ...
... d. Most of our cholesterol is converted into bile salts and acids e. The carbons in cholesterol can be traced to carbohydrates ...
electron transport chain
... Respiration During respiration, electrons are shuttled through electron carriers to a final electron acceptor. aerobic respiration: final electron receptor is oxygen (O2) anaerobic respiration: final electron acceptor is an inorganic molecule (not O2) fermentation: final electron acceptor is an org ...
... Respiration During respiration, electrons are shuttled through electron carriers to a final electron acceptor. aerobic respiration: final electron receptor is oxygen (O2) anaerobic respiration: final electron acceptor is an inorganic molecule (not O2) fermentation: final electron acceptor is an org ...
Chapter 6
... involves bond breaking and bond forming • The initial energy needed to start a chemical reaction is called the free energy of activation, or activation energy (EA) • Activation energy is often supplied in the form of heat from the surroundings ...
... involves bond breaking and bond forming • The initial energy needed to start a chemical reaction is called the free energy of activation, or activation energy (EA) • Activation energy is often supplied in the form of heat from the surroundings ...
Cellular Respiration: Harvesting Chemical Energy
... CoA breaks off to gather more acetic acid. The Acetic acid is broken down. ...
... CoA breaks off to gather more acetic acid. The Acetic acid is broken down. ...
AP Biology Ch. 9 Fermentation and Quiz Ppt
... Proteins: Broken down to amino acids—these go to either glycolysis or the Citric Acid Cycle Fats: Broken down to glycerol (goes to glycolysis) and fatty acids (made into Acetyl CoA). ...
... Proteins: Broken down to amino acids—these go to either glycolysis or the Citric Acid Cycle Fats: Broken down to glycerol (goes to glycolysis) and fatty acids (made into Acetyl CoA). ...
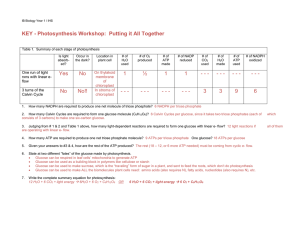
KEY - Photosynthesis Workshop: Putting it All Together
... Glucose can be used to make ALL the biomolecules plant cells need: amino acids (also requires N), fatty acids, nucleotides (also requires N), etc. ...
... Glucose can be used to make ALL the biomolecules plant cells need: amino acids (also requires N), fatty acids, nucleotides (also requires N), etc. ...
Chem331 Krebs Cycle
... • Szent-Gyorgyi determined the catalytic affect of small amounts of future TCA intermediates • Knoop (also key in fatty acid metabolism) the formation of citrate form OAA and Pyruvate • Krebs found a cycle of reforming catalytic amount of oxaloacetate The Krebs cycle is a central pathway for rec ...
... • Szent-Gyorgyi determined the catalytic affect of small amounts of future TCA intermediates • Knoop (also key in fatty acid metabolism) the formation of citrate form OAA and Pyruvate • Krebs found a cycle of reforming catalytic amount of oxaloacetate The Krebs cycle is a central pathway for rec ...
Regulation of fatty acid oxidation in cells
... ketone bodies are also made from acetyl-CoA derived from the catabolism of some amino acids and pyruvate oxidation, representing perhaps 10-18% of the amount made from fatty acids [14]. T h e oxidation of one molecule of palmitate to eight acetyl-CoA molecules consumes 14 atoms of oxygen, whereas it ...
... ketone bodies are also made from acetyl-CoA derived from the catabolism of some amino acids and pyruvate oxidation, representing perhaps 10-18% of the amount made from fatty acids [14]. T h e oxidation of one molecule of palmitate to eight acetyl-CoA molecules consumes 14 atoms of oxygen, whereas it ...
Lehninger Principles of Biochemistry 5/e
... 1. In animal, AA undergo oxidative degradation in three different metabolic circumstance - The normal degradation of cellular protein - A diet is rich in protein - When carbohydrates are either unavailable, cellular proteins are used as fuel 2.AA lose their amino groups to form a-keto acid 3.a-keto ...
... 1. In animal, AA undergo oxidative degradation in three different metabolic circumstance - The normal degradation of cellular protein - A diet is rich in protein - When carbohydrates are either unavailable, cellular proteins are used as fuel 2.AA lose their amino groups to form a-keto acid 3.a-keto ...
Basal metabolic rate

Basal metabolic rate (BMR) is the minimal rate of energy expenditure per unit time by endothermic animals at rest. (McNab, B. K. 1997). On the Utility of Uniformity in the Definition of Basal Rate of Metabolism. Physiol. Zool. Vol.70; Metabolism refers to the processes that the body needs to function. Basal Metabolic Rate is the amount of energy expressed in calories that a person needs to keep the body functioning at rest. Some of those processes are breathing, blood circulation, controlling body temperature, cell growth, brain and nerve function, and contraction of muscles. Basal metabolic rate (BMR) affects the rate that a person burns calories and ultimately whether you maintain, gain, or lose weight. Your basal metabolic rate accounts for about 60 to 75% of the calories you burn every day. It is influenced by several factors.