Efficient Homogeneous Catalysis in the Reduction of CO to CO
... toward CO2. Metal boryls often display distinctive reactivity,15 catalyzing a number of remarkable transformations.16 Although C-B bond-forming reactions have been achieved using diboron compounds with catalytic17a or stoichiometric17b copper(I), welldefined copper boryl complexes have not been desc ...
... toward CO2. Metal boryls often display distinctive reactivity,15 catalyzing a number of remarkable transformations.16 Although C-B bond-forming reactions have been achieved using diboron compounds with catalytic17a or stoichiometric17b copper(I), welldefined copper boryl complexes have not been desc ...
Full Text - Verlag der Zeitschrift für Naturforschung
... The calculations carried out in this work indicate that the structures 1a (cyclobutenyne) and 1c (tetrahedrene) do not represent minima on the potential energy surface (PES) for C 4 H2 , in agreement with the literature [14]. The structure 1b was already proposed previously to be stable [15]; its en ...
... The calculations carried out in this work indicate that the structures 1a (cyclobutenyne) and 1c (tetrahedrene) do not represent minima on the potential energy surface (PES) for C 4 H2 , in agreement with the literature [14]. The structure 1b was already proposed previously to be stable [15]; its en ...
Sodium acetate method for determining CEC of cadmium
... 3.6%. Organic matter was the main contributor to CEC; but in this investigation, the organic bonding fraction represented only 2.3%. Most of the Cd that was bound to organic matter was plausibly extracted in the exchangeable fraction. Figures 1 and 2 present the distributions of each fraction of Cd ...
... 3.6%. Organic matter was the main contributor to CEC; but in this investigation, the organic bonding fraction represented only 2.3%. Most of the Cd that was bound to organic matter was plausibly extracted in the exchangeable fraction. Figures 1 and 2 present the distributions of each fraction of Cd ...
CHEMISTRY 314-01 MIDTERM # 1 – answer key February 10, 2009
... One expects three peaks for the molecular ion. Since 79Br and 81Br are equally abundant, the intensity of the M+ and the (M+4)+ should be the same. However, the (M+2)+ signal should have double intensity, because the combination 79Br and 81Br is twice as likely statistically. So the ratio of M+ : (M ...
... One expects three peaks for the molecular ion. Since 79Br and 81Br are equally abundant, the intensity of the M+ and the (M+4)+ should be the same. However, the (M+2)+ signal should have double intensity, because the combination 79Br and 81Br is twice as likely statistically. So the ratio of M+ : (M ...
Polar Alignment of -Shaped Basic Building Units within Transition
... bond ij, and R0 and B are empirical constants dependent on the identity of the i and j bonded elements. Bond valence values arrived at by this method are not applicable for bonds to disordered sites or sites of partial occupancy, and such bonds are not included in our calculations. ...
... bond ij, and R0 and B are empirical constants dependent on the identity of the i and j bonded elements. Bond valence values arrived at by this method are not applicable for bonds to disordered sites or sites of partial occupancy, and such bonds are not included in our calculations. ...
Synthesis and Nuclear Magnetic Resonance Spectra of ISN
... In the absence of a routine N M R probe, X-ray crystallography has been the only reliable method for establishing the molecular structure of sulfur-nitrogen compounds. Mason and cu-workers2ahave reported 14Nchemical shifts for a variety of N-sulfinylamines and N-sulfenamides and for S4N4,2b but the ...
... In the absence of a routine N M R probe, X-ray crystallography has been the only reliable method for establishing the molecular structure of sulfur-nitrogen compounds. Mason and cu-workers2ahave reported 14Nchemical shifts for a variety of N-sulfinylamines and N-sulfenamides and for S4N4,2b but the ...
Nuclear Magnetic Resonance: The Organic
... Recall that approximately half of Hx are “spin up” and half are “spin down” (random distribution). The same can be said for Hy. Thus, half of the sample will have the magnetic field from Hy aligned with the external magnetic field, deshielding Hx. The other half of the sample will have the magnetic ...
... Recall that approximately half of Hx are “spin up” and half are “spin down” (random distribution). The same can be said for Hy. Thus, half of the sample will have the magnetic field from Hy aligned with the external magnetic field, deshielding Hx. The other half of the sample will have the magnetic ...
Fragmentations Associated with Organic Functional Groups
... ketones, cleavage usually occurs on both sides of the carbonyl function, the more abundant cleavage reflects the relative stabilities of both the acylium cation and the radical. Also, carbonyl compounds that have a hydrogen atom on a carbon three atoms away from the carbonyl carbon (i.e. a -hydroge ...
... ketones, cleavage usually occurs on both sides of the carbonyl function, the more abundant cleavage reflects the relative stabilities of both the acylium cation and the radical. Also, carbonyl compounds that have a hydrogen atom on a carbon three atoms away from the carbonyl carbon (i.e. a -hydroge ...
Binuclear Metal Complexes of a Doubly Bridged Cyclopentadienyl
... polymers that exhibit interesting electric, magnetic, and optical properties.2 Secondly, the metal centers may show cooperative chemical effecta which have, for example, been envisaged to be useful for homogeneous ~ a t a l y s i s . ~ Flexible bridges impede interactions of both types, because thes ...
... polymers that exhibit interesting electric, magnetic, and optical properties.2 Secondly, the metal centers may show cooperative chemical effecta which have, for example, been envisaged to be useful for homogeneous ~ a t a l y s i s . ~ Flexible bridges impede interactions of both types, because thes ...
containing complexes of aromatic amino acids
... are attributable to the dissociation of [Phe+H]+;24 the former has been assigned as the a1 or iminium ion, H2N+QCHCH2C6H5, the latter the benzyl cation, C6H5CH2+. However, dissociation of [Phe+H]+ is also expected to give a prominent product ion at m/z 103, which is absent; in addition, the abundant ...
... are attributable to the dissociation of [Phe+H]+;24 the former has been assigned as the a1 or iminium ion, H2N+QCHCH2C6H5, the latter the benzyl cation, C6H5CH2+. However, dissociation of [Phe+H]+ is also expected to give a prominent product ion at m/z 103, which is absent; in addition, the abundant ...
1H NMR
... In general we will only be using the data in an IR spectrum for stretching vibrations which have energies higher than 1620 cm-1. Although the bands at lower energy are known and assigned, the region below 1620 cm-1 is very congested with single bond stretches of two heavy atoms (see C-C and C-O in t ...
... In general we will only be using the data in an IR spectrum for stretching vibrations which have energies higher than 1620 cm-1. Although the bands at lower energy are known and assigned, the region below 1620 cm-1 is very congested with single bond stretches of two heavy atoms (see C-C and C-O in t ...
[HMIM][Br9]: a Room-temperature Ionic Liquid Based on a
... In view of its slightly lower conductivity, when compared with the quaternary ammonia salts, the main advantage of the present system is based on its liquid nature at room temperature. This will potentially permit its application as a liquid redox-active electrode. NMR measurements According to Span ...
... In view of its slightly lower conductivity, when compared with the quaternary ammonia salts, the main advantage of the present system is based on its liquid nature at room temperature. This will potentially permit its application as a liquid redox-active electrode. NMR measurements According to Span ...
Developing Lewis structures for organic molecules 1) Draw the full
... • like all orbitals, MOs can hold two electrons at most. • MOs can be delocalized - where atomic orbitals are found around an atom, molecular orbitals can be spread over the entire molecule • MOs can be σ or π • molecular orbitals can be bonding, non-bonding or anti-bonding • a chart showing the rel ...
... • like all orbitals, MOs can hold two electrons at most. • MOs can be delocalized - where atomic orbitals are found around an atom, molecular orbitals can be spread over the entire molecule • MOs can be σ or π • molecular orbitals can be bonding, non-bonding or anti-bonding • a chart showing the rel ...
Synthetic Strategy – Lecture 2 (DC, 19.1.05)
... Adding an organometallic compound to a carbonyl compound very often generates a new stereocentre, and it’s non-trivial to control which enantiomer of the product is formed. If we disconnect one bond further away from the alcohol in the target, we generate a β hydroxyalkyl cation synthon, whose synth ...
... Adding an organometallic compound to a carbonyl compound very often generates a new stereocentre, and it’s non-trivial to control which enantiomer of the product is formed. If we disconnect one bond further away from the alcohol in the target, we generate a β hydroxyalkyl cation synthon, whose synth ...
Solving Spectroscopy Problems: Putting it All Together Once you`ve
... 3) Since we still have an oxygen left, it can attach to either the CH or the CH3 singlet. Look at the 1H‐NMR chemical shift and you will see that the CH3 singlet has the higher chemical shift, so it is probably attached to the oxygen. So, attach the CH(CH3)2 on one position on the benzene ring a ...
... 3) Since we still have an oxygen left, it can attach to either the CH or the CH3 singlet. Look at the 1H‐NMR chemical shift and you will see that the CH3 singlet has the higher chemical shift, so it is probably attached to the oxygen. So, attach the CH(CH3)2 on one position on the benzene ring a ...
Powerpoint - U of L Class Index
... Each 1H has a spin, so each 1H is generating its own magnetic field. Recall that approximately half of the Hx are “spin up” and half are “spin down” (random distribution). The same can be said for Hy. Thus, half of the sample will have the magnetic field from Hy aligned with the external magnetic fi ...
... Each 1H has a spin, so each 1H is generating its own magnetic field. Recall that approximately half of the Hx are “spin up” and half are “spin down” (random distribution). The same can be said for Hy. Thus, half of the sample will have the magnetic field from Hy aligned with the external magnetic fi ...
PDF Full-text
... Harcourt has found a remarkable way to unite the idea of Bohr orbits with the electron cube structure of Lewis in a three-dimensional theory of the molecular structural formula [22]. Harcourt’s Bohr orbit model is shown side to side with Linnett’s model both for O2 triplet in Figure 2. Both of these ...
... Harcourt has found a remarkable way to unite the idea of Bohr orbits with the electron cube structure of Lewis in a three-dimensional theory of the molecular structural formula [22]. Harcourt’s Bohr orbit model is shown side to side with Linnett’s model both for O2 triplet in Figure 2. Both of these ...
Mass spectroscopy
... Some times ions may also exist with two or three charges instead of usual single charge in the mass spectrum. These are known as doubly or triply charged ions. They are created as follows: M+° + e- M++ + 3eBut under normal operating conditions, most of the ions produced are single charged. The dou ...
... Some times ions may also exist with two or three charges instead of usual single charge in the mass spectrum. These are known as doubly or triply charged ions. They are created as follows: M+° + e- M++ + 3eBut under normal operating conditions, most of the ions produced are single charged. The dou ...
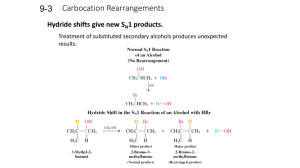
Carbocation Rearrangements
... Rearrangement of an initial secondary carbocation to the more stable tertiary carbocation by a hydride shift results in a rearranged product. ...
... Rearrangement of an initial secondary carbocation to the more stable tertiary carbocation by a hydride shift results in a rearranged product. ...
Reactions of 2, 6-cycloheptadienone and 2, 7
... Yearly 10 years ago, Hor&k2reported the characterization by paper chromatography of tropinone prepared from a large excess of methylamine and 20 mg of a mixture of cycloheptadienones, which had been prepared by treatment of a tropinonium salt with base.3 I n 1965, Garbisch4 described the preparation ...
... Yearly 10 years ago, Hor&k2reported the characterization by paper chromatography of tropinone prepared from a large excess of methylamine and 20 mg of a mixture of cycloheptadienones, which had been prepared by treatment of a tropinonium salt with base.3 I n 1965, Garbisch4 described the preparation ...
Chemical Bonding II
... In ethylene, H2C=CH2, the sp2 orbitals are used to make one of the bonds between the carbon atoms and the bonds between carbon and hydrogen. These bonds have electron density along the internuclear (bond) axis. This type of bond is called a σ (sigma) bond. ...
... In ethylene, H2C=CH2, the sp2 orbitals are used to make one of the bonds between the carbon atoms and the bonds between carbon and hydrogen. These bonds have electron density along the internuclear (bond) axis. This type of bond is called a σ (sigma) bond. ...
2-Norbornyl cation

In organic chemistry, the term 2-norbornyl cation (equivalent with 2-bicyclo-[2.2.1]heptyl cation) describes one of the three carbocations formed from derivatives of norbornane. Though 1-norbornyl and 7-norbornyl cations have been studied, the most extensive studies and vigorous debates have been centered on the exact structure of the 2-norbornyl cation.The 2-norbornyl cation has been formed from a variety of norbornane derivatives and reagents. First reports of its formation and reactivity published by Saul Winstein sparked controversy over the nature of its bonding, as he invoked a three-center two-electron bond to explain the stereoselectivity of the resulting product. Herbert C. Brown challenged this assertion on the grounds that classical resonance structures could explain the stereospecificity without needing to adapt a new perspective of bonding.Evidence of the non-classical nature of the 2-norbornyl cation grew over the course of several decades, mainly through spectroscopic data gathered using methods such as Nuclear magnetic resonance (NMR). Crystallographic confirmation of its non-classical nature did not come until quite recently.The nature of bonding in the 2-norbornyl cation incorporated many new ideas into the field’s understanding of chemical bonds. Similarities can be seen between this cation and others, such as boranes.














![[HMIM][Br9]: a Room-temperature Ionic Liquid Based on a](http://s1.studyres.com/store/data/016911324_1-ac5688316a1e3a6c1ba364df016e5832-300x300.png)