Proton Chemical Shift Tensors and Hydrogen Bond Geometry: A 1H
... chemistry, biochemistry, and biology.1-5 Due to its versatility and applicability to molecular systems in all condensed phases, nuclear magnetic resonance (NMR) spectroscopy is commonly used for studying hydrogen-bonding phenomena. The early discovery of the effect of hydrogen bonding on 1H chemical ...
... chemistry, biochemistry, and biology.1-5 Due to its versatility and applicability to molecular systems in all condensed phases, nuclear magnetic resonance (NMR) spectroscopy is commonly used for studying hydrogen-bonding phenomena. The early discovery of the effect of hydrogen bonding on 1H chemical ...
Evidence for tautomerism in nucleic acid base
... N labeled tRNA appear as asymmetric doublet signals, the asymmetry being dependent on the applied magnetic field strength. Assuming a tautomerism of the type N-H—N =* N--H-N in the base pairs the line shapes can be simulated. The most important parameters fitted in the simulation are the rate consta ...
... N labeled tRNA appear as asymmetric doublet signals, the asymmetry being dependent on the applied magnetic field strength. Assuming a tautomerism of the type N-H—N =* N--H-N in the base pairs the line shapes can be simulated. The most important parameters fitted in the simulation are the rate consta ...
Chapter 8 Lecture
... Rearrangements are evidence for carbocation intermediates and serve to confirm the SN1 reaction mechanism. ...
... Rearrangements are evidence for carbocation intermediates and serve to confirm the SN1 reaction mechanism. ...
6.10 Acid-Catalyzed Hydration of Alkenes
... Thermodynamics of Addition-Elimination Equlibria How do we control the position of the equilibrium and maximize the product? ...
... Thermodynamics of Addition-Elimination Equlibria How do we control the position of the equilibrium and maximize the product? ...
T_AllylCF3paperBM[5]
... Thus, the anhydrous FeCl3 was found to be very effective Lewis acid to achieve the transformations of CF3-substituted allyl alcohols 1 to the corresponding dimers 3 at rt for just 1 hour. It is worth mentioning that these reactions need at least 50 mole % of FeCl3, rather than catalytic one. The use ...
... Thus, the anhydrous FeCl3 was found to be very effective Lewis acid to achieve the transformations of CF3-substituted allyl alcohols 1 to the corresponding dimers 3 at rt for just 1 hour. It is worth mentioning that these reactions need at least 50 mole % of FeCl3, rather than catalytic one. The use ...
Ch04-04-alkenes-2
... Exergonic reaction: early transition state resembles reactants (I). Endergonic reaction: late transition state resembles products (II). ...
... Exergonic reaction: early transition state resembles reactants (I). Endergonic reaction: late transition state resembles products (II). ...
- University at Albany
... Also, alkyl halide reactivity decreases from methyl to 10 to 20 to 30. In fact, 30 alkyl halides do not react by SN2. Leaving group: The substrate should have a good leaving group. A good leaving group should be electron withdrawing, relatively stable, and polarizable. They are weak bases. Examples ...
... Also, alkyl halide reactivity decreases from methyl to 10 to 20 to 30. In fact, 30 alkyl halides do not react by SN2. Leaving group: The substrate should have a good leaving group. A good leaving group should be electron withdrawing, relatively stable, and polarizable. They are weak bases. Examples ...
Chapter 7: Alkene reactions
... Goal: Piece together information from reactions to figure out structures of unknown compounds. You are given some key pieces of information to help you figure out what is happening to the molecule in each step. Example: Compound A has the formula C10H16. On catalytic hydrogenation over palladium (H2 ...
... Goal: Piece together information from reactions to figure out structures of unknown compounds. You are given some key pieces of information to help you figure out what is happening to the molecule in each step. Example: Compound A has the formula C10H16. On catalytic hydrogenation over palladium (H2 ...
Correlated/non-correlated ion dynamics of charge
... The title salt, 3-butyl-1-methyl-1H-imidazolium hexafluorophosphate, [C4mim][PF6] (see Fig. 1), has to date received much attention in the arena of molecular dynamics (MD), usually employing static high field approaches that probe fast motions.9–11 Our interest in this particular salt, which possess ...
... The title salt, 3-butyl-1-methyl-1H-imidazolium hexafluorophosphate, [C4mim][PF6] (see Fig. 1), has to date received much attention in the arena of molecular dynamics (MD), usually employing static high field approaches that probe fast motions.9–11 Our interest in this particular salt, which possess ...
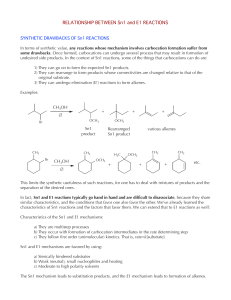
RELATIONSHIP BETWEEN Sn1 and E1 REACTIONS
... to form HBr, which is an inorganic product of the reaction. However, we will not focus on inorganic products. As a matter of fact, inorganic products are frequently left out when writing organic reactions and mechanisms to avoid clutter and keep the focus on the organic products. b) Rearrange to a m ...
... to form HBr, which is an inorganic product of the reaction. However, we will not focus on inorganic products. As a matter of fact, inorganic products are frequently left out when writing organic reactions and mechanisms to avoid clutter and keep the focus on the organic products. b) Rearrange to a m ...
Polymer Electrolytes
... - the chains wrap around cation without excess strain → right spacing of (-CH2-CH2-O-)n unit - other coordination groups: -NR- , -NH- and –S● Interaction strength btn. cation and coordinating group classified according to hard/soft acid base theory (HSAB) → HA prefer HB and SA do SB; e.g., polyether ...
... - the chains wrap around cation without excess strain → right spacing of (-CH2-CH2-O-)n unit - other coordination groups: -NR- , -NH- and –S● Interaction strength btn. cation and coordinating group classified according to hard/soft acid base theory (HSAB) → HA prefer HB and SA do SB; e.g., polyether ...
enzymatic And Limited Industrial Use
... A stereospecific reaction is one in which a single starting material yields only a single stereoisomer ...
... A stereospecific reaction is one in which a single starting material yields only a single stereoisomer ...
(MgCl2 and CaCl2): Osmotic Pressure Calculations
... biomolecules becomes much more important in studies involving the description of conformational changes occurring after ion binding. Mg2+ binding to unstructured RNA molecules is known to shift the folding versus unfolding equilibrium toward folded states.2 Similarly, the activation mechanism of the ...
... biomolecules becomes much more important in studies involving the description of conformational changes occurring after ion binding. Mg2+ binding to unstructured RNA molecules is known to shift the folding versus unfolding equilibrium toward folded states.2 Similarly, the activation mechanism of the ...
PowerPoint ******
... (4) Steric factors may also significantly affect the open-chain cations. Non-migrating substituents are forced into “eclipsed position in the TS for rearrangement. ...
... (4) Steric factors may also significantly affect the open-chain cations. Non-migrating substituents are forced into “eclipsed position in the TS for rearrangement. ...
stereochemistry of internucleotide bond formation by the h
... Ribonucleoside H-phosphonate monoesters show surprisingly high stereoselectivity during condensing agents-promoted formation of an internucleotidic bond1,2. The activation of ribonucleoside H-phosphonates of type 1 with pivaloyl chloride yields two diastereomers (A and B, Fig. 1) of mixed anhydrides ...
... Ribonucleoside H-phosphonate monoesters show surprisingly high stereoselectivity during condensing agents-promoted formation of an internucleotidic bond1,2. The activation of ribonucleoside H-phosphonates of type 1 with pivaloyl chloride yields two diastereomers (A and B, Fig. 1) of mixed anhydrides ...
HCN Synthesis from Methane and Ammonia: Mechanisms of Pt
... CH2NH2+. The isomeric species CH3NH+ and CHNH3+ are assumed to be far less stable and thus excluded from further consideration. The connectivity of the ionic product CH2NH2+ is supported by deuterium labeling in that PtCD2+ and NH3 give solely loss of PtH and formation of CD2NH2+ for channel 3a; the ...
... CH2NH2+. The isomeric species CH3NH+ and CHNH3+ are assumed to be far less stable and thus excluded from further consideration. The connectivity of the ionic product CH2NH2+ is supported by deuterium labeling in that PtCD2+ and NH3 give solely loss of PtH and formation of CD2NH2+ for channel 3a; the ...
The Molecular Structure of Bismuth Oxide by
... strengths, and overall symmetry of metal oxide species. This is not only true for the more common crystalline and solution phases (17), but also for the exotic twodimensional surface phases (18,19). The basic idea behind the Raman analysis is that different molecular structures typically have differ ...
... strengths, and overall symmetry of metal oxide species. This is not only true for the more common crystalline and solution phases (17), but also for the exotic twodimensional surface phases (18,19). The basic idea behind the Raman analysis is that different molecular structures typically have differ ...
A study on the nickel(II)
... coordination for a blue Ni(II)-fam complex. A thiazole nitrogen N(9) and one of the two guanidine nitrogens, N(2) or N(3), are the most likely binding sites. Since at this pH proton dissociation from the ligand does not occur, two anions of ClO 2 4 bind to Ni(II) to compensate for the positive charg ...
... coordination for a blue Ni(II)-fam complex. A thiazole nitrogen N(9) and one of the two guanidine nitrogens, N(2) or N(3), are the most likely binding sites. Since at this pH proton dissociation from the ligand does not occur, two anions of ClO 2 4 bind to Ni(II) to compensate for the positive charg ...
J. Am. Chem. SOC. 1993,115, 7685-7695
... ( C(C5Mes)Rh(PMe3)~DS)H (C6D4H)D.7 Further detailed studies of the isotope effects in both the oxidative addition and the reductive elimination reactions were also interpreted in terms of an q2-benzene intermediate.8 Recently, flash photolysis studies have provided direct evidence for this species p ...
... ( C(C5Mes)Rh(PMe3)~DS)H (C6D4H)D.7 Further detailed studies of the isotope effects in both the oxidative addition and the reductive elimination reactions were also interpreted in terms of an q2-benzene intermediate.8 Recently, flash photolysis studies have provided direct evidence for this species p ...
Multinuclear NMR as a tool for studying local order and
... A complex dynamical picture has progressively emerged for MAPbX3 bulk materials, which combine highly anharmonic lattice vibrations and stochastic MA reorientations, at high temperature. These properties significantly influence the ...
... A complex dynamical picture has progressively emerged for MAPbX3 bulk materials, which combine highly anharmonic lattice vibrations and stochastic MA reorientations, at high temperature. These properties significantly influence the ...
Covalent Bonding What is covalent bonding? Hybrid Orbital Formation
... The notation means that each hybrid is composed of 1/4 s and 3/4 p orbitals. Hybrid orbitals: combinations of atomic orbitals (on one atom). better for bonding (more directed) Mary J. Bojan ...
... The notation means that each hybrid is composed of 1/4 s and 3/4 p orbitals. Hybrid orbitals: combinations of atomic orbitals (on one atom). better for bonding (more directed) Mary J. Bojan ...
Organic Chemistry
... Carbocation: a species in which a carbon atom has only six electrons in its valence shell and bears positive charge Carbocations are • classified as 1°, 2°, or 3° depending on the number of carbons bonded to the carbon bearing the positive charge • electrophiles; that is, they are electron-loving • ...
... Carbocation: a species in which a carbon atom has only six electrons in its valence shell and bears positive charge Carbocations are • classified as 1°, 2°, or 3° depending on the number of carbons bonded to the carbon bearing the positive charge • electrophiles; that is, they are electron-loving • ...
Reversible binding of sulfur dioxide to arylplatinum (II) and nickel (II
... Elemental analyses of the product pointed to a dinuclear compound. In order to get a better understanding of the correlation between structural and physicochemical properties of 2a-f a spectroscopic study was carried out and the structure of one of the complexes was crystallographically studied. IR ...
... Elemental analyses of the product pointed to a dinuclear compound. In order to get a better understanding of the correlation between structural and physicochemical properties of 2a-f a spectroscopic study was carried out and the structure of one of the complexes was crystallographically studied. IR ...
2-Norbornyl cation

In organic chemistry, the term 2-norbornyl cation (equivalent with 2-bicyclo-[2.2.1]heptyl cation) describes one of the three carbocations formed from derivatives of norbornane. Though 1-norbornyl and 7-norbornyl cations have been studied, the most extensive studies and vigorous debates have been centered on the exact structure of the 2-norbornyl cation.The 2-norbornyl cation has been formed from a variety of norbornane derivatives and reagents. First reports of its formation and reactivity published by Saul Winstein sparked controversy over the nature of its bonding, as he invoked a three-center two-electron bond to explain the stereoselectivity of the resulting product. Herbert C. Brown challenged this assertion on the grounds that classical resonance structures could explain the stereospecificity without needing to adapt a new perspective of bonding.Evidence of the non-classical nature of the 2-norbornyl cation grew over the course of several decades, mainly through spectroscopic data gathered using methods such as Nuclear magnetic resonance (NMR). Crystallographic confirmation of its non-classical nature did not come until quite recently.The nature of bonding in the 2-norbornyl cation incorporated many new ideas into the field’s understanding of chemical bonds. Similarities can be seen between this cation and others, such as boranes.


![T_AllylCF3paperBM[5]](http://s1.studyres.com/store/data/003584459_1-3decab572f7fca68901a941affab18ea-300x300.png)