Long Version
... What is a primary producer? What is a primary consumer? What is a heterotroph? autotroph? What is biological magnification? Explain the rule of 10% in regards to trophic levels. What are there not unlimited steps in the energy pyramid? What is primary productivity? What is Gross primary productivity ...
... What is a primary producer? What is a primary consumer? What is a heterotroph? autotroph? What is biological magnification? Explain the rule of 10% in regards to trophic levels. What are there not unlimited steps in the energy pyramid? What is primary productivity? What is Gross primary productivity ...
GHW#10-Questions
... c. Which amino acid gives an acidic solution? d. Which amino acid gives a basic solution? ...
... c. Which amino acid gives an acidic solution? d. Which amino acid gives a basic solution? ...
Practicing with Cladograms
... With advances in molecular biology, scientists are able to take a closer look at similarities among organisms and to look for evolutionary relationships at the molecular level. The amino acid sequence of a protein can be examined in much the same way as the derived traits shown in the previous secti ...
... With advances in molecular biology, scientists are able to take a closer look at similarities among organisms and to look for evolutionary relationships at the molecular level. The amino acid sequence of a protein can be examined in much the same way as the derived traits shown in the previous secti ...
Organic Molecules
... The carbon chain is saturated with all the hydrogens it can hold Account for the solid nature of fats, like butter, at room temperature ...
... The carbon chain is saturated with all the hydrogens it can hold Account for the solid nature of fats, like butter, at room temperature ...
Biochemistry Notes
... 4. Values of n ranging from three to seven are called simple sugars, or monosaccharides. ...
... 4. Values of n ranging from three to seven are called simple sugars, or monosaccharides. ...
What is RNA? - Manhasset Schools
... DNA is too ________________ to leave the nucleus, so a smaller molecule called __________ is made to carry the _______________________ out of the _________________ so ____________________ can be made. * This is completed through the process of _________________________________ * ...
... DNA is too ________________ to leave the nucleus, so a smaller molecule called __________ is made to carry the _______________________ out of the _________________ so ____________________ can be made. * This is completed through the process of _________________________________ * ...
Taken from http://www.gtac.edu.au/ 2007 EXPLORING ENZYME
... EXTENSION QUESTIONS 16. Many plants have a natural inhibitor of this enzyme. For example, beans contain the inhibitor phaseolin. Why would they contain this inhibitor? ...
... EXTENSION QUESTIONS 16. Many plants have a natural inhibitor of this enzyme. For example, beans contain the inhibitor phaseolin. Why would they contain this inhibitor? ...
4 – 2 Chemical Compounds in Living Things
... produced by polymerization where 2 or more monosaccharides (monomers) combine to form larger molecules (polymers) o This process is called DEHYDRATION SYNTHESIS (dehydration=loss of water, synthesis=putting together) Chemical bond that links 2 simple sugars; forms at an –OH group on one sugar and ...
... produced by polymerization where 2 or more monosaccharides (monomers) combine to form larger molecules (polymers) o This process is called DEHYDRATION SYNTHESIS (dehydration=loss of water, synthesis=putting together) Chemical bond that links 2 simple sugars; forms at an –OH group on one sugar and ...
DNA polymerase I
... In E. coli, pol I fills in gaps in the lagging strand and removes RNA primer. Fragments are joined by DNA ...
... In E. coli, pol I fills in gaps in the lagging strand and removes RNA primer. Fragments are joined by DNA ...
Use of Reduced Carbon Compounds
... Entner-Doudoroff pathway (ED) --- variation of gylcolysis produces only 1 net ATP but also 1 NADPH ...
... Entner-Doudoroff pathway (ED) --- variation of gylcolysis produces only 1 net ATP but also 1 NADPH ...
from innovative technologies ...to superior key products
... F R O M I N N O VAT I V E T E C H N O L O G I E S . . . Nucleic Acid Testing Nucleic acids store and transfer genetic information in cells. The main types of nucleic acids are DNA and R NA, which are made up of chains of chemicals called nucleotides. Most DNA exists in cells as a double-stranded str ...
... F R O M I N N O VAT I V E T E C H N O L O G I E S . . . Nucleic Acid Testing Nucleic acids store and transfer genetic information in cells. The main types of nucleic acids are DNA and R NA, which are made up of chains of chemicals called nucleotides. Most DNA exists in cells as a double-stranded str ...
Chemistry of Life Review Sheet Key
... 8. Structurally cellulose is very similar to starch. However, cellulose is a much stronger molecule due to the flip flop manner of the bonds linking the glucose monomers. In cellulose there are no SIDE CHAINS which allow the molecules to lie close to one another giving the opportunity for many HYDRO ...
... 8. Structurally cellulose is very similar to starch. However, cellulose is a much stronger molecule due to the flip flop manner of the bonds linking the glucose monomers. In cellulose there are no SIDE CHAINS which allow the molecules to lie close to one another giving the opportunity for many HYDRO ...
Molecules of Life
... Hydrogen and other elements covalently bonded to carbon Four types of organic compounds: ...
... Hydrogen and other elements covalently bonded to carbon Four types of organic compounds: ...
Slide 1
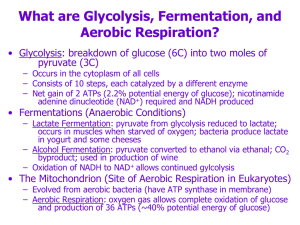
... occurs in muscles when starved of oxygen; bacteria produce lactate in yogurt and some cheeses – Alcohol Fermentation: pyruvate converted to ethanol via ethanal; CO2 byproduct; used in production of wine – Oxidation of NADH to NAD+ allows continued gylcolysis ...
... occurs in muscles when starved of oxygen; bacteria produce lactate in yogurt and some cheeses – Alcohol Fermentation: pyruvate converted to ethanol via ethanal; CO2 byproduct; used in production of wine – Oxidation of NADH to NAD+ allows continued gylcolysis ...
Translation - Advanced
... Proteins (Advanced) concept. Briefly, the primary structure of the protein is the sequence of amino acids determined by the gene and mRNA. The secondary and tertiary structures are determined by interactions between the amino acids within the polypeptide (Figure 1.3). Many proteins undergo post-tran ...
... Proteins (Advanced) concept. Briefly, the primary structure of the protein is the sequence of amino acids determined by the gene and mRNA. The secondary and tertiary structures are determined by interactions between the amino acids within the polypeptide (Figure 1.3). Many proteins undergo post-tran ...
Al - Iraqia university/ college of medicine
... formation of lesions, or atherosclerotic plaques, inside blood vessels. The plaques narrow blood vessel diameter, choking off blood & oxygen supply to tissues. Atherosclerosis is a cause of cardiovascular disease (heart attack & stroke). More harmful than naturally occurring saturated fats are trans ...
... formation of lesions, or atherosclerotic plaques, inside blood vessels. The plaques narrow blood vessel diameter, choking off blood & oxygen supply to tissues. Atherosclerosis is a cause of cardiovascular disease (heart attack & stroke). More harmful than naturally occurring saturated fats are trans ...
File - Biology with Radjewski
... molecules of ATP must be hydrolyzed to start the process 30 molecules of NADH are produced 6 molecules of FADH2 are produced 18 molecules of ATP are produced via substrate phosphorylation (12 in glycolysis and 6 in Krebs) 18 molecules of water are produced in ETS 18 molecules of CO2 are re ...
... molecules of ATP must be hydrolyzed to start the process 30 molecules of NADH are produced 6 molecules of FADH2 are produced 18 molecules of ATP are produced via substrate phosphorylation (12 in glycolysis and 6 in Krebs) 18 molecules of water are produced in ETS 18 molecules of CO2 are re ...
Document
... Chargaff’s rule hydrogen bonding RNA exists as a single stand. – contains ribose instead of deoxyribose – contains uracil in place of thymine ...
... Chargaff’s rule hydrogen bonding RNA exists as a single stand. – contains ribose instead of deoxyribose – contains uracil in place of thymine ...
What is a Protein?
... The basic structure of every amino acid is the same. Each amino acid contains an amino group (-NH2) and a carboxyl group (-COOH). The only difference between one amino acid and the next is the “R” group. “R” represents the “Radical” side chain that is different for each amino acid. The “R” group ca ...
... The basic structure of every amino acid is the same. Each amino acid contains an amino group (-NH2) and a carboxyl group (-COOH). The only difference between one amino acid and the next is the “R” group. “R” represents the “Radical” side chain that is different for each amino acid. The “R” group ca ...
Chapter 17 (part 2) - University of Nevada, Reno
... ubiquitin marks that protein for destruction ...
... ubiquitin marks that protein for destruction ...
Biosynthesis

Biosynthesis (also called biogenesis or anabolism) is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined together to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides.The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g.NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide bonds, and DNA molecules, which are composed of nucleotides joined via phosphodiester bonds.