Prof. Kamakaka`s Lecture 14 Notes (PPT)
... Hexokinase activity increases with increased glucose but activity is inhibited by increased G6P. The levels of enzyme are constitutive. It only generates ATP when energy is required. Glucokinase is not normally active because its Km is lower than normal blood glucose levels. Eating food increases gl ...
... Hexokinase activity increases with increased glucose but activity is inhibited by increased G6P. The levels of enzyme are constitutive. It only generates ATP when energy is required. Glucokinase is not normally active because its Km is lower than normal blood glucose levels. Eating food increases gl ...
Slide 1
... • Non-amino acid chemical groups can enhance protein function – “Prosthetic groups” “Enzyme co-factor” – Associated or covalently-bound – eg. Metals • Iron, Calcium, Zinc, Magnesium, etc. • Structural components • Good nucleophiles: enzymatic ‘activation’ of water, for example • Redox chemistry: acc ...
... • Non-amino acid chemical groups can enhance protein function – “Prosthetic groups” “Enzyme co-factor” – Associated or covalently-bound – eg. Metals • Iron, Calcium, Zinc, Magnesium, etc. • Structural components • Good nucleophiles: enzymatic ‘activation’ of water, for example • Redox chemistry: acc ...
Enzymes
... A. Enzymes provide a site where reactants can be brought together to react. B. The reactants of enzyme-catalyzed reactions are known as substrates. C. The substrates bind to a site on the enzyme called the active site. D. The fit between the enzyme and its substrate are so precise that it is often c ...
... A. Enzymes provide a site where reactants can be brought together to react. B. The reactants of enzyme-catalyzed reactions are known as substrates. C. The substrates bind to a site on the enzyme called the active site. D. The fit between the enzyme and its substrate are so precise that it is often c ...
MEMBRANE-BOUND ELECTRON TRANSFER AND ATP
... Chemotrophs derive energy from oxidation of fuel molecules and in aerobic organisms the ultimate electron acceptor is O2 Electron is not transferred directly Electron is transferred through special carriers, Pyridine nucleotides Electron acceptor ...
... Chemotrophs derive energy from oxidation of fuel molecules and in aerobic organisms the ultimate electron acceptor is O2 Electron is not transferred directly Electron is transferred through special carriers, Pyridine nucleotides Electron acceptor ...
lecture CH23 chem131pikul
... •The electron transport chain provides the energy to pump H+ ions across the inner membrane of the mitochondria. •The concentration of H+ ions in the inter membrane space becomes higher than that inside the matrix creating a potential energy gradient. •To return to the matrix, H+ ions travel through ...
... •The electron transport chain provides the energy to pump H+ ions across the inner membrane of the mitochondria. •The concentration of H+ ions in the inter membrane space becomes higher than that inside the matrix creating a potential energy gradient. •To return to the matrix, H+ ions travel through ...
Chapter 14: Carbohydrates
... Proteins Proteins form components of the body such as muscles, hair, and nails Enzymes are proteins that act as tiny “machines” in cellular processes Hemoglobin is a protein that carries oxygen in the blood Proteins can also act as storage molecules. Some hormones are proteins. ...
... Proteins Proteins form components of the body such as muscles, hair, and nails Enzymes are proteins that act as tiny “machines” in cellular processes Hemoglobin is a protein that carries oxygen in the blood Proteins can also act as storage molecules. Some hormones are proteins. ...
Biological Energy Systems
... – In general, there is an inverse relationship between a given energy system’s maximum rate of ATP production (i.e., ATP produced per unit of time) and the total amount of ATP it is capable of producing over a long period. – As a result, the phosphagen energy system primarily supplies ATP for high-i ...
... – In general, there is an inverse relationship between a given energy system’s maximum rate of ATP production (i.e., ATP produced per unit of time) and the total amount of ATP it is capable of producing over a long period. – As a result, the phosphagen energy system primarily supplies ATP for high-i ...
Cells
... contents in prokaryotic cells are contained within the cytoplasm. The function of the cytoplasm is to provide support and physical structure while also acting like a medium for transport inside the cell. The jelly-like substance allows organelles to "float" freely throughout the cell. It acts like a ...
... contents in prokaryotic cells are contained within the cytoplasm. The function of the cytoplasm is to provide support and physical structure while also acting like a medium for transport inside the cell. The jelly-like substance allows organelles to "float" freely throughout the cell. It acts like a ...
Respiration - Ms. Killikelly's Science Classes
... Substrate-Level Phosphorylation Oxidative Phosphorylation ...
... Substrate-Level Phosphorylation Oxidative Phosphorylation ...
Reactions
... producing more substrate than is consumed – Works as long as the produced triose phosphate is NOT diverted elsewhere (as in times of stress or disease) ...
... producing more substrate than is consumed – Works as long as the produced triose phosphate is NOT diverted elsewhere (as in times of stress or disease) ...
9/2/08 Transcript I - UAB School of Optometry
... Utilized in "Fight or Flight"- If confronted by a lion then you will fight or flee and use this type of process because it does not require any set up time or oxygen. There are 10 rxns which are the same in all cells, but may not happen at same rate. 2 Phases: 1. Converts glucose to two Glycer ...
... Utilized in "Fight or Flight"- If confronted by a lion then you will fight or flee and use this type of process because it does not require any set up time or oxygen. There are 10 rxns which are the same in all cells, but may not happen at same rate. 2 Phases: 1. Converts glucose to two Glycer ...
Amino Acids and Proteins: →Protein Functions: enzymes, transport
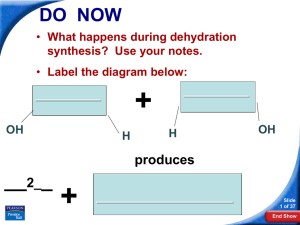
... Polymerization of Amino Acids into Peptides and Proteins Polymerization in cells is catalyzed by enzymes associated with the ribosomes. It is simply a dehydration reaction (see figure 4.15). The lone pairs on the nitrogen of one amino acid perform a nucleophilic attack on the carbonyl of the other a ...
... Polymerization of Amino Acids into Peptides and Proteins Polymerization in cells is catalyzed by enzymes associated with the ribosomes. It is simply a dehydration reaction (see figure 4.15). The lone pairs on the nitrogen of one amino acid perform a nucleophilic attack on the carbonyl of the other a ...
Y13 Biology Y2 PLCs Student Teacher 1
... in a series of oxidation-reduction reactions, the Krebs cycle generates reduced coenzymes and ATP by substrate-level phosphorylation, and carbon dioxide is lost synthesis of ATP by oxidative phosphorylation is associated with the transfer of electrons down the electron transfer chain and passage ...
... in a series of oxidation-reduction reactions, the Krebs cycle generates reduced coenzymes and ATP by substrate-level phosphorylation, and carbon dioxide is lost synthesis of ATP by oxidative phosphorylation is associated with the transfer of electrons down the electron transfer chain and passage ...
Answers to Review Questions
... other types of molecules chemically bonded to it. These proteins may then pass to other structures of the cell when they are enclosed in vesicles that bud from the RER. Further modification may occur in the smooth ER and/or the Golgi complex. Ultimately, these membrane bound vesicles containing thes ...
... other types of molecules chemically bonded to it. These proteins may then pass to other structures of the cell when they are enclosed in vesicles that bud from the RER. Further modification may occur in the smooth ER and/or the Golgi complex. Ultimately, these membrane bound vesicles containing thes ...
04b AP Bio The Structure and Function of Proteins and Nucleic
... • Amino acids are linked by peptide bonds • A polypeptide is a polymer of amino acids • Polypeptides range in length from a few to more than a thousand monomers (Yikes!) • Each polypeptide has a unique linear sequence of amino acids, with a carboxyl end (C-terminus) and an amino end (N-terminus) ...
... • Amino acids are linked by peptide bonds • A polypeptide is a polymer of amino acids • Polypeptides range in length from a few to more than a thousand monomers (Yikes!) • Each polypeptide has a unique linear sequence of amino acids, with a carboxyl end (C-terminus) and an amino end (N-terminus) ...
ch9sec1n2_2013
... INTERMEMBRANE SPACE represents _______________________ potential energy that is harnessed to make ATP. As H+ ions escape through ion channels ATP SYNTHASE back into the matrix, ________________ spins and adds a phosphate to ADP to ATP form _______ ...
... INTERMEMBRANE SPACE represents _______________________ potential energy that is harnessed to make ATP. As H+ ions escape through ion channels ATP SYNTHASE back into the matrix, ________________ spins and adds a phosphate to ADP to ATP form _______ ...
The Structure and Function of Macromolecules
... • Primary structure consists of its unique sequence of amino acids • Secondary structure, found in most proteins, consists of coils and folds in the polypeptide chain • Tertiary structure is determined by interactions among various side chains (R groups) • Quaternary structure results when a protein ...
... • Primary structure consists of its unique sequence of amino acids • Secondary structure, found in most proteins, consists of coils and folds in the polypeptide chain • Tertiary structure is determined by interactions among various side chains (R groups) • Quaternary structure results when a protein ...
04b AP Bio The Structure and Function of Proteins and Nucleic
... • Amino acids are linked by peptide bonds • A polypeptide is a polymer of amino acids • Polypeptides range in length from a few to more than a thousand monomers (Yikes!) • Each polypeptide has a unique linear sequence of amino acids, with a carboxyl end (C-terminus) and an amino end (N-terminus) ...
... • Amino acids are linked by peptide bonds • A polypeptide is a polymer of amino acids • Polypeptides range in length from a few to more than a thousand monomers (Yikes!) • Each polypeptide has a unique linear sequence of amino acids, with a carboxyl end (C-terminus) and an amino end (N-terminus) ...
Inborn Errors of Metabolism A Hospitalist`s Approach
... E to F. C ispath notD.present the enzyme make Bthat to C is defective, pathways to Also, if the apoenzyme and cofactors form the enzyme of reactions are moot (transport defect) B accumulates anddefective, further shunts down alternate pathways to D. converting B to C are B backs up and diverts down ...
... E to F. C ispath notD.present the enzyme make Bthat to C is defective, pathways to Also, if the apoenzyme and cofactors form the enzyme of reactions are moot (transport defect) B accumulates anddefective, further shunts down alternate pathways to D. converting B to C are B backs up and diverts down ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.