Chemical Evolution of Life on the Early Earth All organisms on Earth
... can get 5 H2CO + heat --> ribose sugar, and 5 HCN (hydrogen cyanide) ---> adenine (a base of nucleic acids). There have been hundreds of such “gas-discharge” experiments since then. They have produced not only many of the amino acids used by life, but also the four bases (A, C, G, U) used by RNA and ...
... can get 5 H2CO + heat --> ribose sugar, and 5 HCN (hydrogen cyanide) ---> adenine (a base of nucleic acids). There have been hundreds of such “gas-discharge” experiments since then. They have produced not only many of the amino acids used by life, but also the four bases (A, C, G, U) used by RNA and ...
Name: ______ Date: Period: ATP, Photosynthesis and Cellular
... What is Cellular Respiration? http://www.biology.iupui.edu/biocourses/N100/2k4ch7respirationnotes.html 29. What is the definition of Cellular Respiration?(in purple) 30. What happens during cellular respiration? 31. What’s the equation for Cellular Respiration? Stages of Cellular respiration. http: ...
... What is Cellular Respiration? http://www.biology.iupui.edu/biocourses/N100/2k4ch7respirationnotes.html 29. What is the definition of Cellular Respiration?(in purple) 30. What happens during cellular respiration? 31. What’s the equation for Cellular Respiration? Stages of Cellular respiration. http: ...
Unit 2 Objectives - Chemistry of Life
... 1.2 Describe the basic molecular structures and primary functions of the four major categories of organic molecules (carbohydrates, lipids, proteins, nucleic acids). 1.3 Explain the role of enzymes as catalysts that lower the activation energy of biochemical reactions. Identify factors, such as pH a ...
... 1.2 Describe the basic molecular structures and primary functions of the four major categories of organic molecules (carbohydrates, lipids, proteins, nucleic acids). 1.3 Explain the role of enzymes as catalysts that lower the activation energy of biochemical reactions. Identify factors, such as pH a ...
NUTRICALM A Formula Designed to Calm and Sooth NutriCalm
... NUTRICALM A Formula Designed to Calm and Sooth NutriCalm features pharmaceutical grade L-tryptophan, an essential amino acid which is converted to serotonin in the brain. In addition, the herbs ashwaganda, theanine and valerian root help soothe and relax naturally, effectively and safely. 1 Capsule ...
... NUTRICALM A Formula Designed to Calm and Sooth NutriCalm features pharmaceutical grade L-tryptophan, an essential amino acid which is converted to serotonin in the brain. In addition, the herbs ashwaganda, theanine and valerian root help soothe and relax naturally, effectively and safely. 1 Capsule ...
Nutrient cycles - VBIOLOGY
... coenzyme A to produce acetylcoenzyme A (acetyl CoA). Another oxidation reaction occurs when NAD+ collects more hydrogen ions. This forms reduced NAD (NADH + H+) No ATP is produced in this reaction. ...
... coenzyme A to produce acetylcoenzyme A (acetyl CoA). Another oxidation reaction occurs when NAD+ collects more hydrogen ions. This forms reduced NAD (NADH + H+) No ATP is produced in this reaction. ...
Synaptic Transmission
... •- synthesized from oxygen and the amino acid arginine. Not stored in vesicles. •- diffuses into its target cell rather than a membrane bound receptor and binds to proteins and nucleic acids. •- it has a half-life of about 2-30s. • - For example, NO is synthesized in the endothelial lining of blood ...
... •- synthesized from oxygen and the amino acid arginine. Not stored in vesicles. •- diffuses into its target cell rather than a membrane bound receptor and binds to proteins and nucleic acids. •- it has a half-life of about 2-30s. • - For example, NO is synthesized in the endothelial lining of blood ...
Fatty acids - Haverford Alchemy
... The liver synthesizes glycogen from glucose, glucose from noncarbohydrate precursors, triacylglycerols from mono- and diacylglycerols, fatty acids, cholesterol, bile acids, plasma proteins, and blood clotting factors, and can catabolize glucose, fatty acids, and amino acids. The liver stores glycoge ...
... The liver synthesizes glycogen from glucose, glucose from noncarbohydrate precursors, triacylglycerols from mono- and diacylglycerols, fatty acids, cholesterol, bile acids, plasma proteins, and blood clotting factors, and can catabolize glucose, fatty acids, and amino acids. The liver stores glycoge ...
Mitochondrial Shuttles and Transporters - Rose
... include small, uncharged molecules (e.g., CO2, O2, and NH3), and some small carboxylic acids, probably in their uncharged forms (e.g., protonated acetic acid). Otherwise, only molecules that have specific transporter proteins are capable of crossing the mitochondrial membrane. ATP/ADP and Phosphate ...
... include small, uncharged molecules (e.g., CO2, O2, and NH3), and some small carboxylic acids, probably in their uncharged forms (e.g., protonated acetic acid). Otherwise, only molecules that have specific transporter proteins are capable of crossing the mitochondrial membrane. ATP/ADP and Phosphate ...
Introduction to Enzymes - Rose
... Although RNA can have catalytic activity, proteins have much more diverse chemistry, because proteins are comprised of 20 amino acids instead of only four nucleotides. In addition, the amino acids have several functional groups that are not present in nucleotides. The chemistry of the amino acid sid ...
... Although RNA can have catalytic activity, proteins have much more diverse chemistry, because proteins are comprised of 20 amino acids instead of only four nucleotides. In addition, the amino acids have several functional groups that are not present in nucleotides. The chemistry of the amino acid sid ...
18_Energy metabolism. Biological oxidation. Chemiosmotic theory
... Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers. ...
... Oxidative phosphorylation is the process in which ATP is formed as a result of the transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers. ...
3.27.12 lecture protein
... Glu + NH3 + ATP Gln • Glutmate synthase -ketoglutarate + glutamine + NADPH2 2 Glu High affinity for NH3 - Concentrates NH3 in cells – Uses ATP Because of N recycling this reaction may not be that important ...
... Glu + NH3 + ATP Gln • Glutmate synthase -ketoglutarate + glutamine + NADPH2 2 Glu High affinity for NH3 - Concentrates NH3 in cells – Uses ATP Because of N recycling this reaction may not be that important ...
Lecture 6
... The 2 ATP’s produced during glycolysis are only a small fraction of the potential energy available from glucose. Under anaerobic conditions, animals convert glucose into 2 molecules of lactate. Much of the potential energy of the glucose molecule remains untapped. Under Aerobic conditions a much mor ...
... The 2 ATP’s produced during glycolysis are only a small fraction of the potential energy available from glucose. Under anaerobic conditions, animals convert glucose into 2 molecules of lactate. Much of the potential energy of the glucose molecule remains untapped. Under Aerobic conditions a much mor ...
Cellular Energy and Enzymatic Function
... • Substrates bind to active site on enzyme • Binding induces conformational change in enzyme--better ”fit” for substrate • Active sites are highly specific and ...
... • Substrates bind to active site on enzyme • Binding induces conformational change in enzyme--better ”fit” for substrate • Active sites are highly specific and ...
Biochemistry 6/e
... Cytoplasmic NADH produced in glycolysis can enter to mitochondria by shuttles glyceraldehyde-3P dehydrogenase in glycolysis ...
... Cytoplasmic NADH produced in glycolysis can enter to mitochondria by shuttles glyceraldehyde-3P dehydrogenase in glycolysis ...
What amino acids really look like
... When an amino acid is incorporated into a polypeptide by the ribosome at position i in the sequence, it undergoes a condensation reaction in which the carboxyl group of the preceding amino acid (i-1) forms an amide (or peptide) bond with the amino group residue i. In the next elongation cycle of the ...
... When an amino acid is incorporated into a polypeptide by the ribosome at position i in the sequence, it undergoes a condensation reaction in which the carboxyl group of the preceding amino acid (i-1) forms an amide (or peptide) bond with the amino group residue i. In the next elongation cycle of the ...
UNIT 11. CATABOLISM OF GLUCOSE • Aerobic glycolysis: scheme
... Glycolysis is an oxidative specific pathway by which one mole of glucose is enzymatically split into two moles of pyruvate. It occurs in cytosol of all cells of the body. The principle function of glycolysis is the generation of ATP. Glycolysis also provides precursors for fatty acids biosynthesis, ...
... Glycolysis is an oxidative specific pathway by which one mole of glucose is enzymatically split into two moles of pyruvate. It occurs in cytosol of all cells of the body. The principle function of glycolysis is the generation of ATP. Glycolysis also provides precursors for fatty acids biosynthesis, ...
Word
... Page 2 of this exam contains information that may be useful to you: (a) abbreviations for the amino acids; (b) pKa values of functional groups; and (c) table of logarithms. A simple calculator is supplied for your use during this exam. No other electronic or computational devices are to be used. ...
... Page 2 of this exam contains information that may be useful to you: (a) abbreviations for the amino acids; (b) pKa values of functional groups; and (c) table of logarithms. A simple calculator is supplied for your use during this exam. No other electronic or computational devices are to be used. ...
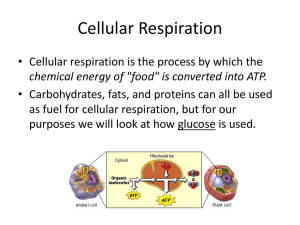
Cellular Respiration
... Aerobic Respiration • Aerobic Respiration has three distinct parts: – Glycolysis occurs in the cytoplasm (yields 2 ATP) – Krebs cycle takes place in the matrix of the mitochondria (yields 2 ATP) – Electron transport chain is carried out on the inner mitochondrial membrane (yields 34 ATP) ...
... Aerobic Respiration • Aerobic Respiration has three distinct parts: – Glycolysis occurs in the cytoplasm (yields 2 ATP) – Krebs cycle takes place in the matrix of the mitochondria (yields 2 ATP) – Electron transport chain is carried out on the inner mitochondrial membrane (yields 34 ATP) ...
Nutritional Requirements and Biosynthetic
... Metabolic studies of the Trypanosomidae have in general been confined to studies of catabolism and there is little known about the pathways of biosynthesis in these organisms. The development of a simple chemically defined growth medium for the parasitic flagellate Strigomonas (Herpetomonas) oncopel ...
... Metabolic studies of the Trypanosomidae have in general been confined to studies of catabolism and there is little known about the pathways of biosynthesis in these organisms. The development of a simple chemically defined growth medium for the parasitic flagellate Strigomonas (Herpetomonas) oncopel ...
CHM_224_201510 - Oakton Community College
... reactions, polar reactions and the interconversion of resonance structures. 9. Rationalize the regioselectivity, stereoselectivity, chemoselectivity and reactivity of chemical reactions. ...
... reactions, polar reactions and the interconversion of resonance structures. 9. Rationalize the regioselectivity, stereoselectivity, chemoselectivity and reactivity of chemical reactions. ...
Metabolism

Metabolism (from Greek: μεταβολή metabolē, ""change"") is the set of life-sustaining chemical transformations within the cells of living organisms. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to all chemical reactions that occur in living organisms, including digestion and the transport of substances into and between different cells, in which case the set of reactions within the cells is called intermediary metabolism or intermediate metabolism.Metabolism is usually divided into two categories: catabolism, the breaking down of organic matter by way of cellular respiration, and anabolism, the building up of components of cells such as proteins and nucleic acids. Usually, breaking down releases energy and building up consumes energy.The chemical reactions of metabolism are organized into metabolic pathways, in which one chemical is transformed through a series of steps into another chemical, by a sequence of enzymes. Enzymes are crucial to metabolism because they allow organisms to drive desirable reactions that require energy that will not occur by themselves, by coupling them to spontaneous reactions that release energy. Enzymes act as catalysts that allow the reactions to proceed more rapidly. Enzymes also allow the regulation of metabolic pathways in response to changes in the cell's environment or to signals from other cells.The metabolic system of a particular organism determines which substances it will find nutritious and which poisonous. For example, some prokaryotes use hydrogen sulfide as a nutrient, yet this gas is poisonous to animals. The speed of metabolism, the metabolic rate, influences how much food an organism will require, and also affects how it is able to obtain that food.A striking feature of metabolism is the similarity of the basic metabolic pathways and components between even vastly different species. For example, the set of carboxylic acids that are best known as the intermediates in the citric acid cycle are present in all known organisms, being found in species as diverse as the unicellular bacterium Escherichia coli and huge multicellular organisms like elephants. These striking similarities in metabolic pathways are likely due to their early appearance in evolutionary history, and their retention because of their efficacy.

![Glycolysis [Compatibility Mode]](http://s1.studyres.com/store/data/015804080_1-177318d0ac1e37e7516326c4d927443b-300x300.png)