
Slide 1
... oWhen an e- occupies an orbit greater than the lowest possible energy level it is said to be in an “excited state” oΔE=-Rhc(1/nf2 - 1/ni2) Rhc=1312 kJ/mol Wave/particle duality oTaken from idea that light, usually considered to exhibit wave properties, actually consists of particles (photons) oSim ...
... oWhen an e- occupies an orbit greater than the lowest possible energy level it is said to be in an “excited state” oΔE=-Rhc(1/nf2 - 1/ni2) Rhc=1312 kJ/mol Wave/particle duality oTaken from idea that light, usually considered to exhibit wave properties, actually consists of particles (photons) oSim ...
Introduction to Chemistry
... To describe how diffraction experiments were used to demonstrate the dual nature of all matter. To show that the line spectrum of hydrogen demonstrates the quanitzed nature of the energy of its electron. To describe the development of the Bohr model for the hydrogen atom. To show how standing waves ...
... To describe how diffraction experiments were used to demonstrate the dual nature of all matter. To show that the line spectrum of hydrogen demonstrates the quanitzed nature of the energy of its electron. To describe the development of the Bohr model for the hydrogen atom. To show how standing waves ...
Single Spin Asymmetries with real photons in inclusive eN scattering
... possible for one circular and other non-circular planets to emerge ...
... possible for one circular and other non-circular planets to emerge ...
Chapter 6.2 Notes: Use Proportions to Solve Geometry Problems
... Ex.7: You buy a 3-D scale model of the Reunion Tower in Dallas, TX. The actual building is 560 feet tall. Your model is 10 inches tall, and the diameter of the dome on your scale model is about 2.1 inches. a. What is the diameter of the actual dome? b. About how many times as tall as your model is ...
... Ex.7: You buy a 3-D scale model of the Reunion Tower in Dallas, TX. The actual building is 560 feet tall. Your model is 10 inches tall, and the diameter of the dome on your scale model is about 2.1 inches. a. What is the diameter of the actual dome? b. About how many times as tall as your model is ...
Path integral in quantum mechanics
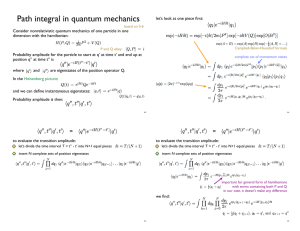
... calculate only time-ordered products of Qs, and if H is no more than quadratic in P and if the term quadratic in P does not involve Q, then the equation above can be written as: ...
... calculate only time-ordered products of Qs, and if H is no more than quadratic in P and if the term quadratic in P does not involve Q, then the equation above can be written as: ...
Thermodynamics and Statistical Mechanics I - Home Exercise 4
... (a) What are the possible energies of a single spin? (b) What is the partition function of the system? (c) Find the average magnetization M in the z direction. Express it using Langevin’s function: L(x) = coth(x) − x1 . (d) Expand this result for small x, and in addition find the limit of Langevin’s ...
... (a) What are the possible energies of a single spin? (b) What is the partition function of the system? (c) Find the average magnetization M in the z direction. Express it using Langevin’s function: L(x) = coth(x) − x1 . (d) Expand this result for small x, and in addition find the limit of Langevin’s ...
CH-103 Tutorial-1
... electron in a one dimensional box of length of 1.0 nm. 10. For the particle in a box given in the above question, what is the probability of finding the electron between (i) x = 0.49 and 0.51, (ii) x = 0 and 0.020 and (ii) x=0.24 and 0.26 ( x in nm) for both n=1 and n=2. Rationalize your answers. 11 ...
... electron in a one dimensional box of length of 1.0 nm. 10. For the particle in a box given in the above question, what is the probability of finding the electron between (i) x = 0.49 and 0.51, (ii) x = 0 and 0.020 and (ii) x=0.24 and 0.26 ( x in nm) for both n=1 and n=2. Rationalize your answers. 11 ...
Precursors to Modern Physics
... Antiquity: mostly philosophical and mathematical discussions. Physics in Middle Ages: many physics concepts like gravity, momentum started to form. Early modern or classical physics: mid 16th to late 18th centuries: physics as a science was well established by experimental measurements and observati ...
... Antiquity: mostly philosophical and mathematical discussions. Physics in Middle Ages: many physics concepts like gravity, momentum started to form. Early modern or classical physics: mid 16th to late 18th centuries: physics as a science was well established by experimental measurements and observati ...
Precursors to Modern Physics
... Antiquity: mostly philosophical and mathematical discussions. Physics in Middle Ages: many physics concepts like gravity, momentum started to form. Early modern or classical physics: mid 16th to late 18th centuries: physics as a science was well established by experimental measurements and observati ...
... Antiquity: mostly philosophical and mathematical discussions. Physics in Middle Ages: many physics concepts like gravity, momentum started to form. Early modern or classical physics: mid 16th to late 18th centuries: physics as a science was well established by experimental measurements and observati ...
A Guided Tour of the Universe
... Quantum mechanics is the basis of all of modern physics, and has been so since the 1920s ...
... Quantum mechanics is the basis of all of modern physics, and has been so since the 1920s ...
A path towards quantum gravity
... supplementary conditions, since the second functional derivative of S is not an invertible operator. After imposing such conditions, the theories are ruled by a differential operator of D’Alembert (or Laplace) type, or a non-minimal operator at the very worst. ...
... supplementary conditions, since the second functional derivative of S is not an invertible operator. After imposing such conditions, the theories are ruled by a differential operator of D’Alembert (or Laplace) type, or a non-minimal operator at the very worst. ...
Syllabus :
... the Newton formulation of mechanics and of basic electromagnetism and thermodynamics from introductory physics courses. ...
... the Newton formulation of mechanics and of basic electromagnetism and thermodynamics from introductory physics courses. ...
INTRODUCTION TO QUANTUM MECHANICS I I mention in class
... Let us make a crude model of a + H2 molecule (that is two protons and one electron). Imagine the protons are held at fixed positions. The electron can be bound to each one of the protons, so we approximate the Hilbert space by a two-dimensional space. When the electron is bound to one proton it has ...
... Let us make a crude model of a + H2 molecule (that is two protons and one electron). Imagine the protons are held at fixed positions. The electron can be bound to each one of the protons, so we approximate the Hilbert space by a two-dimensional space. When the electron is bound to one proton it has ...
Wavefunctions and Bound Systems
... Wavefunction “psi” solves Schroedinger’s equation and contains, in its components, all of the information we need to determine values of observables… ...
... Wavefunction “psi” solves Schroedinger’s equation and contains, in its components, all of the information we need to determine values of observables… ...
Renormalization group

In theoretical physics, the renormalization group (RG) refers to a mathematical apparatus that allows systematic investigation of the changes of a physical system as viewed at different distance scales. In particle physics, it reflects the changes in the underlying force laws (codified in a quantum field theory) as the energy scale at which physical processes occur varies, energy/momentum and resolution distance scales being effectively conjugate under the uncertainty principle (cf. Compton wavelength).A change in scale is called a ""scale transformation"". The renormalization group is intimately related to ""scale invariance"" and ""conformal invariance"", symmetries in which a system appears the same at all scales (so-called self-similarity). (However, note that scale transformations are included in conformal transformations, in general: the latter including additional symmetry generators associated with special conformal transformations.)As the scale varies, it is as if one is changing the magnifying power of a notional microscope viewing the system. In so-called renormalizable theories, the system at one scale will generally be seen to consist of self-similar copies of itself when viewed at a smaller scale, with different parameters describing the components of the system. The components, or fundamental variables, may relate to atoms, elementary particles, atomic spins, etc. The parameters of the theory typically describe the interactions of the components. These may be variable ""couplings"" which measure the strength of various forces, or mass parameters themselves. The components themselves may appear to be composed of more of the self-same components as one goes to shorter distances.For example, in quantum electrodynamics (QED), an electron appears to be composed of electrons, positrons (anti-electrons) and photons, as one views it at higher resolution, at very short distances. The electron at such short distances has a slightly different electric charge than does the ""dressed electron"" seen at large distances, and this change, or ""running,"" in the value of the electric charge is determined by the renormalization group equation.


















![[30 pts] While the spins of the two electrons in a hydrog](http://s1.studyres.com/store/data/002487557_1-ac2bceae20801496c3356a8afebed991-300x300.png)



