BeaniumIsotopeLab
... 2. Explain any differences between the atomic mass of your BEANIUM sample and that of a different lab group. Explain why the difference would be smaller if larger samples were used. 3. What is an isotope? 4. What is the relationship between an element’s isotopes and the element’s atomic mass? 5. Ele ...
... 2. Explain any differences between the atomic mass of your BEANIUM sample and that of a different lab group. Explain why the difference would be smaller if larger samples were used. 3. What is an isotope? 4. What is the relationship between an element’s isotopes and the element’s atomic mass? 5. Ele ...
11. Reactions of Alkyl Halides
... • Instead of halides they used tosylates (OTos) which are better “leaving groups” than halides. • (alkyl toluene sulfonates) ...
... • Instead of halides they used tosylates (OTos) which are better “leaving groups” than halides. • (alkyl toluene sulfonates) ...
NAME GRADED: LET IT BEGIN!!! ____ / 30 pts DIRECTIONS: Use
... Necessary Background: When an isotope is a nuclear radioactive isotope, it means that it can spontaneously breakdown, by emitting alpha particles (effectively He-4 nuclei each equaling 2 protons and 2 neutrons, and of course, 0 electrons), beta particles (high speed e- from degenerating neutrons) or ...
... Necessary Background: When an isotope is a nuclear radioactive isotope, it means that it can spontaneously breakdown, by emitting alpha particles (effectively He-4 nuclei each equaling 2 protons and 2 neutrons, and of course, 0 electrons), beta particles (high speed e- from degenerating neutrons) or ...
Non-Heme Iron Catalyzed Oxidation of Alkanes to Alcohols via
... norbornane), and arenes (benzene, toluene) in the presence of MPPH, will be highlighted. In each case, quantification of the MPPH end product showed clean heterolytic O-O bond cleavage with concomitant oxygen atom transfer efficiencies of > 98%. The catalytic oxidation of methane to methanol at ambi ...
... norbornane), and arenes (benzene, toluene) in the presence of MPPH, will be highlighted. In each case, quantification of the MPPH end product showed clean heterolytic O-O bond cleavage with concomitant oxygen atom transfer efficiencies of > 98%. The catalytic oxidation of methane to methanol at ambi ...
Enrichment of Germanium-73 with the Magnetic Isotope Effect
... Here, &*/A0 and A * / A represent the magnetic isotope abundance obtained before and after photolysis. The isotope selection coefficient,a,is a very important parameter governing the isotope enrichment of starting compound. The isotope enrichment, E, can be estimated from the 6(=Ge) value. In Figure ...
... Here, &*/A0 and A * / A represent the magnetic isotope abundance obtained before and after photolysis. The isotope selection coefficient,a,is a very important parameter governing the isotope enrichment of starting compound. The isotope enrichment, E, can be estimated from the 6(=Ge) value. In Figure ...
Nucleophilic Substitution Reaction
... unstabilized carbanion, it is reasonable to presume if some part is formed it must be either rapidly reconverted to the substrate or converted to the product alkene. ...
... unstabilized carbanion, it is reasonable to presume if some part is formed it must be either rapidly reconverted to the substrate or converted to the product alkene. ...
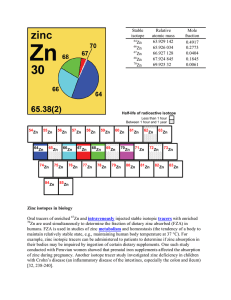
Zinc isotopes in biology Oral tracers of enriched Zn and
... maintain relatively stable state, e.g., maintaining human body temperature at 37 °C). For example, zinc isotopic tracers can be administered to patients to determine if zinc absorption in their bodies may be impaired by ingestion of certain dietary supplements. One such study conducted with Peruvian ...
... maintain relatively stable state, e.g., maintaining human body temperature at 37 °C). For example, zinc isotopic tracers can be administered to patients to determine if zinc absorption in their bodies may be impaired by ingestion of certain dietary supplements. One such study conducted with Peruvian ...
Плеханов В
... studied experimentally from solutions with different content of Cu (65Cu/63Cu) and Zn (66Zn/64Zn). The heavy isotope adsorbed better on the hydroxide surface which is related to a shorter metal-oxygen electron bonds and to a lesser metal coordination number on a surface respectively to dissolved ion ...
... studied experimentally from solutions with different content of Cu (65Cu/63Cu) and Zn (66Zn/64Zn). The heavy isotope adsorbed better on the hydroxide surface which is related to a shorter metal-oxygen electron bonds and to a lesser metal coordination number on a surface respectively to dissolved ion ...
LAB- Beanium_CP Chemistry
... This lab exercise is designed to show you isotopes of an element in a simulation form. You will be asked to gather data about the “isotopes” and organize the data. If atoms were as large as beans they could be sorted, counted, and massed. In this experiment we will sort, count, and mass three differ ...
... This lab exercise is designed to show you isotopes of an element in a simulation form. You will be asked to gather data about the “isotopes” and organize the data. If atoms were as large as beans they could be sorted, counted, and massed. In this experiment we will sort, count, and mass three differ ...
AVERAGE ATOMIC MASS LAB
... contracted to neighboring Arlington High School. Many chemistry students have generously volunteered their time and expertise to help with the followup experiments involving the new element. Dr. Julius Hibbert says the first follow-up experiments conducted at Arlington High School will determine how ...
... contracted to neighboring Arlington High School. Many chemistry students have generously volunteered their time and expertise to help with the followup experiments involving the new element. Dr. Julius Hibbert says the first follow-up experiments conducted at Arlington High School will determine how ...
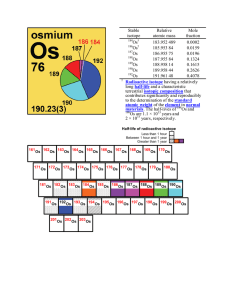
Stable isotope Relative atomic mass Mole fraction Os 183.952 489
... number of protons in the nucleus of an atom is the atomic number. radioactive decay – the process by which unstable (or radioactive) isotopes lose energy by emitting alpha particles (helium nuclei), beta particles (positive or negative electrons), gamma radiation, neutrons or protons to reach a fina ...
... number of protons in the nucleus of an atom is the atomic number. radioactive decay – the process by which unstable (or radioactive) isotopes lose energy by emitting alpha particles (helium nuclei), beta particles (positive or negative electrons), gamma radiation, neutrons or protons to reach a fina ...
Ionic And Covalent Bonds
... c. Be able to calculate the average atomic mass of a set of isotopes. d. Be able to draw a picture (or write a clear description) that represents each different model of the atom. e. Know the difference between an atom, ion, and isotope. f. Be able to count the number of protons, neutrons, and elect ...
... c. Be able to calculate the average atomic mass of a set of isotopes. d. Be able to draw a picture (or write a clear description) that represents each different model of the atom. e. Know the difference between an atom, ion, and isotope. f. Be able to count the number of protons, neutrons, and elect ...
Basic Background Review: Acid-Base , Redox, and Stable Isotopes
... P) have multiple stable isotopes. 2. Within this group, the light isotope (L) is consistently more abundant than the heavy (H) counterpart(s). 3. It is very small (ppt) differences in (H/L) that constitute the basis of using stable isotope signatures as geochemical source and process indicato ...
... P) have multiple stable isotopes. 2. Within this group, the light isotope (L) is consistently more abundant than the heavy (H) counterpart(s). 3. It is very small (ppt) differences in (H/L) that constitute the basis of using stable isotope signatures as geochemical source and process indicato ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... Alkyl Halides React with Nucleophiles and Bases Alkyl halides are polarized at the carbon-halide bond, ...
... Alkyl Halides React with Nucleophiles and Bases Alkyl halides are polarized at the carbon-halide bond, ...
MASS-INDEPENDENT ISOTOPE FRACTIONATION OF CHROMIUM
... in a chemical exchange system [2]. Since then, researchers have applied the new Bigeleisen theory to explain other unusual isotope effects (see, e.g. [3] and references therein). How such mass independent isotopic fractionation would affect interpretations of isotopic anomalies observed in the primi ...
... in a chemical exchange system [2]. Since then, researchers have applied the new Bigeleisen theory to explain other unusual isotope effects (see, e.g. [3] and references therein). How such mass independent isotopic fractionation would affect interpretations of isotopic anomalies observed in the primi ...
Abstract
... Elements are characterized by the number of protons and electrons, i. e., oxygen has eight protons and electrons. Atom core consists of protons and neutrons. It is common that atom core contains variable number of neutrons and constant number of protons. In the case of oxygen, three types of cores a ...
... Elements are characterized by the number of protons and electrons, i. e., oxygen has eight protons and electrons. Atom core consists of protons and neutrons. It is common that atom core contains variable number of neutrons and constant number of protons. In the case of oxygen, three types of cores a ...
Mass Spectrum – Interpretation
... Because you have 79Br-79Br, 81Br-79Br and 81Br-81Br – and if you work out the probablities this is what you would expect. ...
... Because you have 79Br-79Br, 81Br-79Br and 81Br-81Br – and if you work out the probablities this is what you would expect. ...