* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download LAB- Beanium_CP Chemistry
Survey
Document related concepts
Transcript
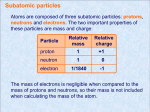
NAME _______________________________________PERIOD____ LAB: Element 119 – Beanium CP Chemistry Introduction & Purpose: What is an isotope? What does it mean to say that the atoms in a sample of an element are isotopes of each other? Ordinary beans are a lot bigger than atoms, but perhaps they can give you one or two clues about isotopes. We have talked in class about isotopes: atoms of the same element that differ in mass. For example, there are actually three different kinds of hydrogen atoms. Refer to the table below for the different kinds (or isotopes) of two common atoms. ISOTOPE Protium Deuterium Tritium Carbon-12 Carbon-14 PROTONS ELECTRONS 1 1 1 1 1 1 6 6 6 6 NEUTRONS 0 1 2 6 8 SIGNIFICANCE “normal” hydrogen “heavy hydrogen” radioactive hydrogen “normal” carbon carbon-14 dating This lab exercise is designed to show you isotopes of an element in a simulation form. You will be asked to gather data about the “isotopes” and organize the data. If atoms were as large as beans they could be sorted, counted, and massed. In this experiment we will sort, count, and mass three different kinds of beans and imagine that we are observing different isotopes of the same element (let’s call it BEANIUM). Each pair of 2 students will work with one sample of three different isotopes. Finally, we will calculate the isotopic mass, the isotopic abundance, and the atomic mass of the bean element. These experiments and calculations are equivalent to the way scientists actually determine the atomic mass of elements. As in real elements, the mixture of isotopes are collections of atoms of the element each having different masses because they have different numbers of neutrons. Unlike real isotopes, the individual isotopic particles of Beanium differ slightly in mass, so you will determine the average mass of each type of isotopic particle. Then you can calculate the "weighted average atomic mass" of Beanium. Definitions/ reference: • Isotopic mass – the average mass of the atoms of a specific isotope of an element • Isotopic abundance – what percent of the element’s atoms are a specific isotope • Atomic mass – the average mass of an element’s atoms #Protons: Determine which element an atom is – determines the atomic number # electrons: Determine the charge on an atom Protons = electrons ………. atom is neutral Protons > electrons ………. atom is positively charged (lost electrons) Protons < electrons ……….. atom is negatively charged (gained electrons) # neutrons: Determines the atomic mass of an atom. Determines the isotope number of the atom. Isotope: An atom of an element with a certain number of neutrons. NOTE: All atoms of an element are isotopes of that element. Most elements have 1, 2 or 3 naturally occurring isotopes. This means that in any sample of the element these naturally occurring isotopes are all present typically always in the same % ratio. For example: The element carbon has three isotopes: = 6 neutrons and is called Carbon -12 isotope = 7 neutrons and is called carbon-13 isotope = 8 neutrons and is called carbon-14 isotope 90% abundance 9% abundance 1% abundance % abundance means that in a sample of carbon (like a lump of coal or a diamond) 90% of the carbon atoms will be carbon-12, 9% will be carbon-13 and 1% will be carbon-14. Since not all the atoms in a sample of an element have the same mass, we have to calculate an average atomic mass for the element. The average atomic mass is calculated taking into account the different percents of each isotope present. __________________________________________________________________________________________________ Purpose: To determine the atomic weight of an element by analogy. Materials: weigh boat, beans, balance Procedure: 1. Using a small plastic cup, take a small sample of beans to create your beanium atoms. The difference between the atom’s isotopes is very distinct. Each bean represents an isotope of the element beanium. Sort the beanium atoms into groups, each group representing a different isotope (by color). Record the total number of atoms (beans) in your sample as well as the number of each type. 2. Find the mass of each isotope to the nearest 0.01g and record in the data table. Return the beans (atoms) to the container carefully. 3. Note: Some beanium atoms have undergone natural fission. Please see instructor for a replacement. Data Table: Bean Type Pink Red Green TOTAL: Number of Beans Mass of Beans Average mass of bean Percent of bean isotope isotope (Isotopic Mass) (Isotopic Abundance) Analysis: 1. Determine the mass of a single beanium atom for each isotope (bean type) by dividing the total mass of each isotope by the number of atoms in that group. This will require three different equations SHOWING WORK! 2. Determine the percent abundance for each isotope by dividing the number of atoms of each isotope by the total number of atoms in the container and multiplying by 100. This will require three different equations SHOWING WORK! 3. Determine the average weighted mass of the element beanium based on the percent abundance of each isotope (#2) and its atomic weight (#1). This will require one equation SHOWING WORK! Use the following formula: Average Weighted Mass = (% as a decimal of isotope A)(mass of one atom of isotope A) + (% as a decimal of isotope B)(mass of one atom of isotope B) + (% as a decimal of isotope C)(mass of one atom of isotope C)… 4. As a check on your answer #3, divide the total sample mass of all beanium isotopes from the container by the total number of beanium isotopes from the container. This answer should match the answer from #3. Conclusion: 5. What do the three kinds of beans represent in this exercise? 6. What do isotopes have in common? How do isotopes differ? 7. What is the difference between percent abundance and relative abundance? 8. Because isotopes have identical chemical properties, radioactive isotopes can be used for medical use. Based on where the following elements are likely found in the body, match each radioisotope with its medical use. (research for answers) _____Sodium-24 a. study of bone formation _____Calcium-47 b. red blood cell studies _____Iodine-131 c. diagnose thyroid disorders _____Iron-55 d measure extracellular fluid _____Phosphorus- 32 e. genetic (DNA) research