
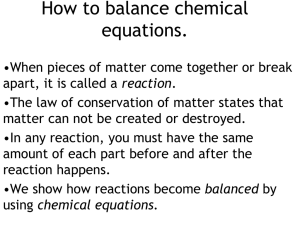
How to balance chemical equations.
... equations. •Multiply the number of atoms you have by coefficients to balance equations. •Multiply coefficients by every subscript until you hit a + or a . •Never, ever, EVER change a subscript. •Make a list of how much of each atom you have and change it as you add coefficients to your equation. •I ...
... equations. •Multiply the number of atoms you have by coefficients to balance equations. •Multiply coefficients by every subscript until you hit a + or a . •Never, ever, EVER change a subscript. •Make a list of how much of each atom you have and change it as you add coefficients to your equation. •I ...
Chapter 2 - Cloudfront.net
... Use the general characteristics of each to distinguish a mixture from a substance. This is the harder way to go. Fixed composition = Substance Varied composition = Mixture We can physically separate mixtures into their component parts. We cannot do so with ...
... Use the general characteristics of each to distinguish a mixture from a substance. This is the harder way to go. Fixed composition = Substance Varied composition = Mixture We can physically separate mixtures into their component parts. We cannot do so with ...
Chapter One Powerpoint - Geneva Area City Schools
... definite shape. • liquid state, matter has a definite volume but an indefinite shape. • gas state, matter has neither definite volume nor definite shape. • Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. ...
... definite shape. • liquid state, matter has a definite volume but an indefinite shape. • gas state, matter has neither definite volume nor definite shape. • Plasma is a high-temperature physical state of matter in which atoms lose most of their electrons, particles that make up atoms. ...
Ch. 8 Notes (Chemical Reactions) Teacher 2010
... Example of a Balanced Chemical Equation: 2H2 (g) + O2 (g) 2H2O (g) left side of the arrow, and the Reactants are on the ______ ...
... Example of a Balanced Chemical Equation: 2H2 (g) + O2 (g) 2H2O (g) left side of the arrow, and the Reactants are on the ______ ...
Intro to Chem
... ◦ Physical methods that are used to separate mixtures cannot be used to break a compound into simpler substances. ◦ Chemical change is a change that produces matter with a different composition than the orginal matter. Sugar broken down into C and H2O(g) when heated. Broken down into H2 and O2 b ...
... ◦ Physical methods that are used to separate mixtures cannot be used to break a compound into simpler substances. ◦ Chemical change is a change that produces matter with a different composition than the orginal matter. Sugar broken down into C and H2O(g) when heated. Broken down into H2 and O2 b ...
Chemical Properties - Michigan State University
... The task is relevant to the objective in the sense that it’s a gateway to learning about chemical processes. In order to be able to explain chemical processes, students must first be able to explain chemical changes. The chemical change concepts will then help the students make connections to chemic ...
... The task is relevant to the objective in the sense that it’s a gateway to learning about chemical processes. In order to be able to explain chemical processes, students must first be able to explain chemical changes. The chemical change concepts will then help the students make connections to chemic ...
Skill Sheet 19-B Chemical Formulas
... Have you ever heard of sodium nitrate? It’s a preservative used in foods like hot dogs. The chemical formula for sodium nitrate is NaNO3. How many types of atoms does this compound contain? You are right if you said three: sodium, nitrogen, and oxygen. The nitrogen and oxygen atoms have a shared-ele ...
... Have you ever heard of sodium nitrate? It’s a preservative used in foods like hot dogs. The chemical formula for sodium nitrate is NaNO3. How many types of atoms does this compound contain? You are right if you said three: sodium, nitrogen, and oxygen. The nitrogen and oxygen atoms have a shared-ele ...
Student Expectation
... Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are called “valence electrons” and h ...
... Key Concept 2: Electrons are located outside of the nucleus and arranged by energy levels in the electron cloud. There are a certain number of electrons that each energy level can hold. Key Concept 3: Electrons located in the outermost shell of the electron cloud are called “valence electrons” and h ...
Describing Matter Chapter 2:2 Physical and Chemical Properties
... • Physical Properties~ a property of matter that can be observed or measured without changing the identity of the matter • Physical Change~ a change that affects one or more physical properties of a substance: many are easy to undo • Chemical Properties~ a property of matter that describes a substan ...
... • Physical Properties~ a property of matter that can be observed or measured without changing the identity of the matter • Physical Change~ a change that affects one or more physical properties of a substance: many are easy to undo • Chemical Properties~ a property of matter that describes a substan ...
Student Activity PDF - TI Education
... 3. For each word equation given on page 2.10, use the Chemical Balance tool on page 2.11 to balance the equation and record it in the table. First, write the balanced equation using the element symbols. Record the number of atoms of each element in the reactant (left side) and the products (right si ...
... 3. For each word equation given on page 2.10, use the Chemical Balance tool on page 2.11 to balance the equation and record it in the table. First, write the balanced equation using the element symbols. Record the number of atoms of each element in the reactant (left side) and the products (right si ...
Common Chemical Formula List
... has remained intact, then that can often be balanced first, as it is acts as a single species. The ions NO3- and CO32- are examples of a complex ion. A VERY useful rule is to leave balancing oxygen and hydrogen to the last steps as these elements are often in more than one chemical on each side , an ...
... has remained intact, then that can often be balanced first, as it is acts as a single species. The ions NO3- and CO32- are examples of a complex ion. A VERY useful rule is to leave balancing oxygen and hydrogen to the last steps as these elements are often in more than one chemical on each side , an ...
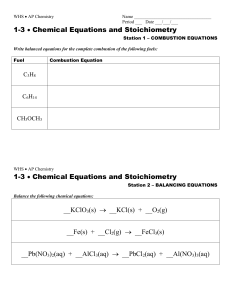
PowerPoint - Balancing Equations
... 4. Check your answer to see if: – The numbers of atoms on both sides of the equation are now balanced. – The coefficients are in the lowest possible whole number ratios. (reduced) ...
... 4. Check your answer to see if: – The numbers of atoms on both sides of the equation are now balanced. – The coefficients are in the lowest possible whole number ratios. (reduced) ...
Chapter 1 Matter and Change
... - can be broken down chemically (not physically) - properties of elements that compounds are broken down into do not resemble properties of original compound ...
... - can be broken down chemically (not physically) - properties of elements that compounds are broken down into do not resemble properties of original compound ...













![[Mg] +2[ S ]-2](http://s1.studyres.com/store/data/014450548_1-468f3af464a09baae245d79fadf97d41-300x300.png)







