How to Balance Chemical Equations
... involved are set and their formulas can not be altered. Hence, any change to the subscripts is NOT allowed. ONLY COEFFICIENTS ARE ALLOWED TO BE CHANGED!! ...
... involved are set and their formulas can not be altered. Hence, any change to the subscripts is NOT allowed. ONLY COEFFICIENTS ARE ALLOWED TO BE CHANGED!! ...
Chapter 3 - Warren County Schools
... broken down by physical or chemical means. – An atom of any element always has the same number of protons. ...
... broken down by physical or chemical means. – An atom of any element always has the same number of protons. ...
Chapter 14, Section 1, pages 494-501
... To describe chemical equilibrium To give examples of chemical equilibrium Demo Burn sulfur in oxygen as an example of a completion reaction. Input Completion Reactions and Reversible Reactions What does reversible mean? Completion Reactions are reactions that use up all or almost all of the reactant ...
... To describe chemical equilibrium To give examples of chemical equilibrium Demo Burn sulfur in oxygen as an example of a completion reaction. Input Completion Reactions and Reversible Reactions What does reversible mean? Completion Reactions are reactions that use up all or almost all of the reactant ...
File Vocabulary PPT set #1
... FAMILIES / GROUPS • Elements that are grouped together based on their chemical properties and reactivity ...
... FAMILIES / GROUPS • Elements that are grouped together based on their chemical properties and reactivity ...
CHEMISTry is life - World of Teaching
... -Too often kids get to high school chemistry and they are scared before they even begin. -My goal is to shape a positive image in their minds about chemistry so that they can be more prepared mentally for high school. -I will do this by showing them how applicable chemistry is to every day life. It ...
... -Too often kids get to high school chemistry and they are scared before they even begin. -My goal is to shape a positive image in their minds about chemistry so that they can be more prepared mentally for high school. -I will do this by showing them how applicable chemistry is to every day life. It ...
Protecting Buildings from Chemical and Biological Warfare Agent
... ASHRAE engineers may use filters and ultraviolet (UV) lamps to remove a variety of biological agents. These measures are not relevant to contain a chemical threat. Furthermore, for a biological threat, filters and UV lamps will usually not suffice to contain the spread of the biological agent from t ...
... ASHRAE engineers may use filters and ultraviolet (UV) lamps to remove a variety of biological agents. These measures are not relevant to contain a chemical threat. Furthermore, for a biological threat, filters and UV lamps will usually not suffice to contain the spread of the biological agent from t ...
chemical bonds - geraldinescience
... Chemical Formulas • A chemical formula is a combination of letters and numbers that shows which elements make up a compound and the number of atoms of each element that are required to make a molecule of a compound. • In a chemical formula, the subscript that appears after the symbol for an element ...
... Chemical Formulas • A chemical formula is a combination of letters and numbers that shows which elements make up a compound and the number of atoms of each element that are required to make a molecule of a compound. • In a chemical formula, the subscript that appears after the symbol for an element ...
Chemical reactions
... equations can be used to concisely represent elements, compounds, and what happens in a chemical reaction. –3 types of formulas ...
... equations can be used to concisely represent elements, compounds, and what happens in a chemical reaction. –3 types of formulas ...
lecture 13
... element must occur on the left (BEFORE the reaction) and on the right (AFTER the reaction) ...
... element must occur on the left (BEFORE the reaction) and on the right (AFTER the reaction) ...
CH 11 Chemical Reaction WS #2 (Pre
... 1. What is the Great Barrier Reef and how was it formed? 2. Define chemical reaction3. How is a chemical reaction different from a physical one? Provide examples to support your explanation. 4. Explain how the appearance of the Statue of Liberty is an example of a chemical reaction: 5. What are stal ...
... 1. What is the Great Barrier Reef and how was it formed? 2. Define chemical reaction3. How is a chemical reaction different from a physical one? Provide examples to support your explanation. 4. Explain how the appearance of the Statue of Liberty is an example of a chemical reaction: 5. What are stal ...
Document
... 2HCl(aq) + Cr(s) H2(g)+ CrCl2(aq) A. composition B. single-displacement C. decomposition D. double-displacement ...
... 2HCl(aq) + Cr(s) H2(g)+ CrCl2(aq) A. composition B. single-displacement C. decomposition D. double-displacement ...
The Language of Chemistry
... more substances in the same phase. No amount of optical magnification will reveal a homogeneous mixture to have different properties in different regions. • A heterogeneous mixture does not have uniform composition. Its components are easily visually distinguishable. • When separated, the components ...
... more substances in the same phase. No amount of optical magnification will reveal a homogeneous mixture to have different properties in different regions. • A heterogeneous mixture does not have uniform composition. Its components are easily visually distinguishable. • When separated, the components ...
Holt Chemistry – Guided Notes, Chapter 1
... • Describe physical and chemical changes, and give examples of each. • Identify the reactants and products in a chemical reaction. • List four observations that suggest a chemical change has occurred. A _______________ is any substance that has a definite composition. A ___________ _______________ i ...
... • Describe physical and chemical changes, and give examples of each. • Identify the reactants and products in a chemical reaction. • List four observations that suggest a chemical change has occurred. A _______________ is any substance that has a definite composition. A ___________ _______________ i ...
1.2 PowerPoint
... Distinguish between physical and chemical properties. Contrast chemical and physical changes. Apply the law of conservation of matter to chemical changes. ...
... Distinguish between physical and chemical properties. Contrast chemical and physical changes. Apply the law of conservation of matter to chemical changes. ...
High School Chemistry Essential Questions
... F. What is the equilibrium model of chemical interactions, what evidence do we have for the equilibrium model of chemical interactions, and how do we use the equilibrium model of chemical interactions to represent, analyze, and communicate structure and relationships in chemical systems and chemical ...
... F. What is the equilibrium model of chemical interactions, what evidence do we have for the equilibrium model of chemical interactions, and how do we use the equilibrium model of chemical interactions to represent, analyze, and communicate structure and relationships in chemical systems and chemical ...
Unit 1 Matter Day 32 2016 Counting Atoms
... Is it balanced? If so, you have supported the Law of Conservation of Matter (Mass). ...
... Is it balanced? If so, you have supported the Law of Conservation of Matter (Mass). ...
Physical and Chemical Changes Worksheet
... An ice cube is placed in the sun. Later there is a puddle of water. Later still the puddle is gone. ...
... An ice cube is placed in the sun. Later there is a puddle of water. Later still the puddle is gone. ...
Physical Properties
... of optical magnification will reveal a homogeneous mixture to have different properties in different regions. • A heterogeneous mixture does not have uniform composition. Its components are easily visually distinguishable. • When separated, the components of both types of mixtures yields pure substa ...
... of optical magnification will reveal a homogeneous mixture to have different properties in different regions. • A heterogeneous mixture does not have uniform composition. Its components are easily visually distinguishable. • When separated, the components of both types of mixtures yields pure substa ...
Science24-UnitA-Section3.4
... Let's first look at simple chemical equations. Read the information in “Math Connect: Checking the Balance of Simple Equations” on page 53 of the textbook. Pay special attention to the steps for checking the balance. 6. Use the sample problem on page 54 of the textbook, 2 H2O(l) → 2H2(g) + O ...
... Let's first look at simple chemical equations. Read the information in “Math Connect: Checking the Balance of Simple Equations” on page 53 of the textbook. Pay special attention to the steps for checking the balance. 6. Use the sample problem on page 54 of the textbook, 2 H2O(l) → 2H2(g) + O ...
chemical reaction - Peoria Public Schools
... Lithium metal and aluminum sulfate solution react to yield a lithium sulfate solution and aluminum metal. ...
... Lithium metal and aluminum sulfate solution react to yield a lithium sulfate solution and aluminum metal. ...
Chemical Reactions
... The reactants are separated from each other by a plus sign and the products are separated from each other by a plus sign. There should be an arrow in the middle. Examples: When sodium is mixed with water, a purple alkaline solution of sodium hydroxide is produced and hydrogen gas is evolved. Sodium ...
... The reactants are separated from each other by a plus sign and the products are separated from each other by a plus sign. There should be an arrow in the middle. Examples: When sodium is mixed with water, a purple alkaline solution of sodium hydroxide is produced and hydrogen gas is evolved. Sodium ...
CLASSROOM CONNECTORS
... A property is a set of identifying characteristics about a substance. Physical properties are easy to identify because they involve your senses of sight, touch, taste, smell and hear. Common examples of physical properties include the color, size and texture of an object. The characteristics, howeve ...
... A property is a set of identifying characteristics about a substance. Physical properties are easy to identify because they involve your senses of sight, touch, taste, smell and hear. Common examples of physical properties include the color, size and texture of an object. The characteristics, howeve ...
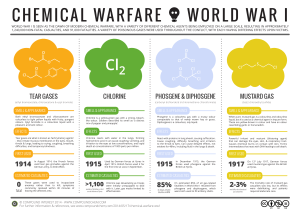
Chemical weapon

A chemical weapon (CW) is a munition that uses chemicals formulated to inflict death or harm on human beings. The Organisation for the Prohibition of Chemical Weapons (OPCW) states: The term chemical weapon may also be applied to any toxic chemical or its precursor that can cause death, injury, temporary incapacitation or sensory irritation through its chemical action. Munitions or other delivery devices designed to deliver chemical weapons, whether filled or unfilled, are also considered weapons themselves.They are classified as weapons of mass destruction (WMDs), though they are distinct from nuclear weapons, biological weapons (diseases), and radiological weapons (which use radioactive decay of elements). All may be used in warfare known by the military acronym NBC, for nuclear, biological, and chemical warfare. Weapons of mass destruction are distinct from conventional weapons, which are primarily effective due to their explosive, kinetic, or incendiary potential. Chemical weapons can be widely dispersed in gas, liquid and solid forms, and may easily afflict others than the intended targets. Nerve gas, tear gas and pepper spray are three modern examples.Lethal, unitary, chemical agents and munitions are extremely volatile and they constitute a class of hazardous chemical weapons that are now being stockpiled by many nations. (Unitary agents are effective on their own and require no mixing with other agents.) The most dangerous of these are nerve agents GA, GB, GD, and VX, and vesicant (blister) agents which are formulations of sulfur mustard such as H, HT, and HD. All are liquids at normal room temperature, but become gaseous when released. Widely used during the First World War, the effects of so-called mustard gas, phosgene gas and others caused lung searing, blindness, death and maiming.Pepper spray is of common use today. It is potentially lethal. There are no recent records of pepper spray being used in war, despite the fact that it inflicts fewer injuries and side-effects compared with impact and explosive weapons.Under the Chemical Weapons Convention (1993), there is a legally binding, world-wide ban on the production, stockpiling, and use of chemical weapons and their precursors. Notwithstanding, large stockpiles thereof continue to exist, usually justified as only a precaution against putative use by an aggressor.