Rotational Motion of Solid Objects
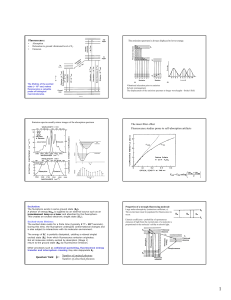
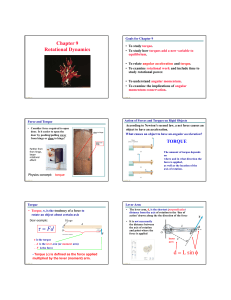
... The units of rotational acceleration are rev/s2 or rad/s2. These definitions for both rotational velocity and rotational acceleration actually yield the average values of these quantities. To get instantaneous values, the time interval t must be made very small, as in the linear-motion definitions o ...
... The units of rotational acceleration are rev/s2 or rad/s2. These definitions for both rotational velocity and rotational acceleration actually yield the average values of these quantities. To get instantaneous values, the time interval t must be made very small, as in the linear-motion definitions o ...
Discharge Generation of Atomic Iodine
... Emission spectroscopy diagnostics Emission spectroscopy is the diagnostic technique widely used for measuring plasma parameters (temperature) and plasma composition i.e. species present in plasma like atoms, molecules and ions. A great advantage of emissive spectroscopy is that it is passive and the ...
... Emission spectroscopy diagnostics Emission spectroscopy is the diagnostic technique widely used for measuring plasma parameters (temperature) and plasma composition i.e. species present in plasma like atoms, molecules and ions. A great advantage of emissive spectroscopy is that it is passive and the ...
Making Sense of Boiling Points and Melting Points
... Molecular symmetry, in our context, can be measured in terms of number of chemically different carbon atoms in the given structures. For example, benzene has six carbon atoms of one single chemical type, unlike toluene, which has seven carbon atoms in five chemically different types – one methyl car ...
... Molecular symmetry, in our context, can be measured in terms of number of chemically different carbon atoms in the given structures. For example, benzene has six carbon atoms of one single chemical type, unlike toluene, which has seven carbon atoms in five chemically different types – one methyl car ...
Fluorescence: Fluorescence studies prone to self
... The amino acids phenylalanine, tyrosine, and tryptophan have -* transitions. There is a pattern of weak bands from 240 - 300 nm and much more intense bands between 190 - 220 nm. The weak bands are allowed by vibronic coupling (L). ...
... The amino acids phenylalanine, tyrosine, and tryptophan have -* transitions. There is a pattern of weak bands from 240 - 300 nm and much more intense bands between 190 - 220 nm. The weak bands are allowed by vibronic coupling (L). ...
AP Physics 1 - Wisconsin Virtual School
... 2. What characteristic of matter causes a gravitational field? 3. Why does the gravitational field between two books on a table not cause them to slide together? 4. What are Kepler's three laws of orbital motion? 5. How can Kepler's third law be derived from Newton's second law? 6. Kepler's second l ...
... 2. What characteristic of matter causes a gravitational field? 3. Why does the gravitational field between two books on a table not cause them to slide together? 4. What are Kepler's three laws of orbital motion? 5. How can Kepler's third law be derived from Newton's second law? 6. Kepler's second l ...
Rotational spectroscopy

Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously.For rotational spectroscopy, molecules are classified according to symmetry into spherical top, linear and symmetric top; analytical expressions can be derived for the rotational energy terms of these molecules. Analytical expressions can be derived for the fourth category, asymmetric top, for rotational levels up to J=3, but higher energy levels need to be determined using numerical methods. The rotational energies are derived theoretically by considering the molecules to be rigid rotors and then applying extra terms to account for centrifugal distortion, fine structure, hyperfine structure and Coriolis coupling. Fitting the spectra to the theoretical expressions gives numerical values of the angular moments of inertia from which very precise values of molecular bond lengths and angles can be derived in favorable cases. In the presence of an electrostatic field there is Stark splitting which allows molecular electric dipole moments to be determined.An important application of rotational spectroscopy is in exploration of the chemical composition of the interstellar medium using radio telescopes.