QM Chemical Shift Calculations to Infer on the Long
... be equal to the sum of individual molecular energies. At such instances the difference in the energy can be termed as an interaction energy, apparently notwithstanding the fact that each molecule had unalterably the same equilibrium geometry all through. For an extraneous proton, it may be convenien ...
... be equal to the sum of individual molecular energies. At such instances the difference in the energy can be termed as an interaction energy, apparently notwithstanding the fact that each molecule had unalterably the same equilibrium geometry all through. For an extraneous proton, it may be convenien ...
Soft X-ray spectroscopy of single sized CdS nanocrystals: size
... into excitonic and unoccupied states. The description of the latter part by a single Gaussian assumes that the vibrational broadening of around 700 meV, as found for the core exciton excitation, masks the fine structure of the CB LPDOS close to the CBM. All fits give the same energy position of the ...
... into excitonic and unoccupied states. The description of the latter part by a single Gaussian assumes that the vibrational broadening of around 700 meV, as found for the core exciton excitation, masks the fine structure of the CB LPDOS close to the CBM. All fits give the same energy position of the ...
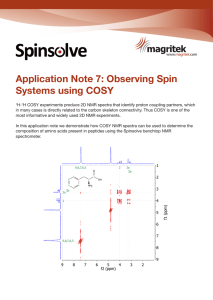
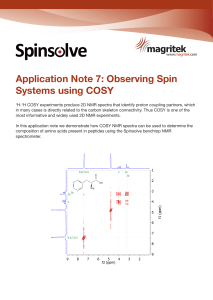
Observing Spin Systems using COSY
... Figure 1: COSY Spectrum of 1,3-dichloro-6-nitro-benzene. Short range coupling is indicated on the spectrum with dashed lines and on the compound with double-headed arrows. The analysis of COSY spectra often allows substantial piecing together of fragments in the molecule as coupling partners and the ...
... Figure 1: COSY Spectrum of 1,3-dichloro-6-nitro-benzene. Short range coupling is indicated on the spectrum with dashed lines and on the compound with double-headed arrows. The analysis of COSY spectra often allows substantial piecing together of fragments in the molecule as coupling partners and the ...
Title: Understanding of Molecular Orbital
... Valence bond theory generally fails to explain the bonding in simple molecules. On the other hand, molecular orbital theory is better approach for the molecules those are having extended π systems. With the help of molecular orbital one can understand the electronic transitions in molecules. ...
... Valence bond theory generally fails to explain the bonding in simple molecules. On the other hand, molecular orbital theory is better approach for the molecules those are having extended π systems. With the help of molecular orbital one can understand the electronic transitions in molecules. ...
Copyright © by Holt, Rinehart and Winston. All rights
... In earlier chapters, the motion of an object was described by assuming the object was a point mass. This description, however, does not account for the differences in the motion of the objects in the race. This is because these objects are extended objects. An extended object is an object that has a ...
... In earlier chapters, the motion of an object was described by assuming the object was a point mass. This description, however, does not account for the differences in the motion of the objects in the race. This is because these objects are extended objects. An extended object is an object that has a ...
View - University of Southampton
... the gas-phase density, the self-consistent field calculation is performed in the presence of the liquid and thus, in general, Fliq(r) * Fgas(r). Therefore, the calculation also includes an electronic polarization contribution. With the addition of analytical first derivatives of eq 2 with respect to ...
... the gas-phase density, the self-consistent field calculation is performed in the presence of the liquid and thus, in general, Fliq(r) * Fgas(r). Therefore, the calculation also includes an electronic polarization contribution. With the addition of analytical first derivatives of eq 2 with respect to ...
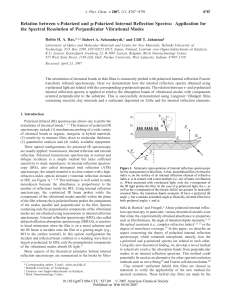
Relation between s-Polarized and p-Polarized Internal Reflection
... Polarized infrared (IR) spectroscopy allows one to probe the orientation of chemical bonds.1-17 The features of polarized IR spectroscopy include (1) simultaneous probing of a wide variety of chemical bonds in organic, inorganic or hybrid materials, (2) sensitivity to measure films down to molecular ...
... Polarized infrared (IR) spectroscopy allows one to probe the orientation of chemical bonds.1-17 The features of polarized IR spectroscopy include (1) simultaneous probing of a wide variety of chemical bonds in organic, inorganic or hybrid materials, (2) sensitivity to measure films down to molecular ...
Transition State Theory
... We shall first consider SN 2 reactions [Substitution, Nucleophilic, 2nd order] because many of these reactions can be described by transition state theory. A Nucleophile is a substance (species) with an unshared electron. It is a species that seeks a positive center. ...
... We shall first consider SN 2 reactions [Substitution, Nucleophilic, 2nd order] because many of these reactions can be described by transition state theory. A Nucleophile is a substance (species) with an unshared electron. It is a species that seeks a positive center. ...
Sequential nonadiabatic excitation of large molecules and ions
... limit, nonadiabatic excitation of atoms can be ignored. However, at shorter wavelengths 共in the optical region兲, nonadiabatic excitation of atoms does occur and may be enhanced and modified by electron correlation effects even for twoelectron atoms 关20兴. For multielectron atoms, large populations of ...
... limit, nonadiabatic excitation of atoms can be ignored. However, at shorter wavelengths 共in the optical region兲, nonadiabatic excitation of atoms does occur and may be enhanced and modified by electron correlation effects even for twoelectron atoms 关20兴. For multielectron atoms, large populations of ...
Rotational spectroscopy

Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy (or just vibronic spectroscopy) where rotational, vibrational and electronic energy changes occur simultaneously.For rotational spectroscopy, molecules are classified according to symmetry into spherical top, linear and symmetric top; analytical expressions can be derived for the rotational energy terms of these molecules. Analytical expressions can be derived for the fourth category, asymmetric top, for rotational levels up to J=3, but higher energy levels need to be determined using numerical methods. The rotational energies are derived theoretically by considering the molecules to be rigid rotors and then applying extra terms to account for centrifugal distortion, fine structure, hyperfine structure and Coriolis coupling. Fitting the spectra to the theoretical expressions gives numerical values of the angular moments of inertia from which very precise values of molecular bond lengths and angles can be derived in favorable cases. In the presence of an electrostatic field there is Stark splitting which allows molecular electric dipole moments to be determined.An important application of rotational spectroscopy is in exploration of the chemical composition of the interstellar medium using radio telescopes.