* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Discharge Generation of Atomic Iodine
Plasma (physics) wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Transition state theory wikipedia , lookup
Rotational–vibrational spectroscopy wikipedia , lookup
Molecular orbital wikipedia , lookup
Chemical bond wikipedia , lookup
Heat transfer physics wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
State of matter wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
Electron configuration wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Chemical imaging wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
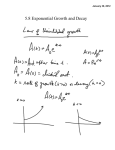
WDS'07 Proceedings of Contributed Papers, Part II, 194–197, 2007. ISBN 978-80-7378-024-1 © MATFYZPRESS Discharge Generation of Atomic Iodine I. Picková, V. Jirásek, J. Schmiedberger Institute of Physics, Academy of Sciences of the Czech republic, Prague, Czech Republic. Abstract. In this article we describe our new experimental device for generation of atomic iodine in discharge. This device will be a part of the COIL (chemical oxygen-iodine laser) and also DOIL (discharge oxygen-iodine laser) system. There are three main tasks which must be solved for a successful COIL operation: generation of atomic iodine, generation of singlet oxygen (excited oxygen molecule) as a source of energy for laser pumping, and efficient mixing of these compounds in the nozzle. Both atomic iodine and singlet oxygen can be produced chemically or in electric discharge. In our laboratory we have started the research of a discharge generation of atomic iodine. For the new discharge method we are going to use the optical emission spectroscopy diagnostics, by which we will detect species present in plasma and use this information for improving of iodine generation process. Introduction A COIL (chemical oxygen iodine laser) is the CW (continuous wave) high power laser radiating at the wavelength 1315 nm (a near infrared region). The word chemical in the name refers to the fact that energy for laser pumping comes from a chemical reaction. This laser radiates on the transition between excited and ground level of atomic iodine: I(2 P1/2 ) + hν → I(2 P3/2 ) + 2hν. (1) Excited iodine atoms are generated from iodine atoms in ground state by collisions with singlet oxygen molecule (first excited state of oxygen - O2 (a1 ∆g )). As the energy distance of the ground and the first excited states of oxygen molecule and iodine atom has nearly the same value, the near-resonant transfer of energy is possible between them, as is shown in the following reaction: I(2 P3/2 ) + O2 (a1 ∆g ) → I(2 P1/2 ) + O2 (a1 Σg ), (2) where I(2 P3/2 ) and I(2 P1/2 ) are the ground and the first excited state of iodine atom respectively, and O2 (a1 ∆g ) and O2 (a1 ∆g ) are the first excited and ground stated of oxygen molecule respectively. There is also a great advantage that O2 (a1 ∆g ) is the metastable state with extremely long radiation lifetime of ∼ 70 minutes [Kodymová et. al., 2004]. Singlet oxygen O2 (a1 ∆g ) is produced in convention COIL by chemical reaction of Cl2 gas and liquid solution of basic hydrogen peroxide (H2 O2 and KOH solution): Cl2 + 2KOH + H2 O2 → O2 (a1 ∆g ) + 2KCl + 2H2 O. (3) In this process it is necessary to achieve the most effective surface reaction area between liquid and gas due to a very short O2 (a1 ∆g ) lifetime in liquid phase (∼ 2µs only). Iodine atoms can be generated from different molecules containing iodine. Classical and still investigated is the iodine molecule I2 , although this molecule has also many disadvantages for the generation process. For dissociation of one I2 molecule, a collision with more than four singlet oxygen molecules is needed, and therefore a part of energy, which could possibly be used for laser pumping, is lost. I2 also quenches excited iodine atoms to the ground state in rather fast reaction. Another disadvantage is that I2 is solid at room temperature and substantial part 194 PICKOVA ET AL.: DISCHARGE GENERATION OF ATOMIC IODINE of device must be heated to avoid its precipitation. For this reasons new source molecules are examined: HI, CH3 I, CF3 I and similar. In our laboratory, a method of chemical generation of atomic iodine from HI molecule is investigated by the substitution mechanism involving atomic chlorine or fluorine [Špalek et. al., 2002, Jirásek et. al., 2001]. This can be simply described by reaction: HI + Cl → HCl + I (4) HI + F → HF + I (5) or Both singlet oxygen and atomic iodine can be generated also in electric discharge. Main advantage of the discharge method is that we can avoid many dangerous chemicals like F2 and Cl2 . Different types of discharge and different molecules containing iodine have been investigated in many laboratories, however improvement of the laser performance by using these methods is still rather limited. In our laboratory we intend to study atomic iodine generation in RF discharge from different iodine donors like I2 , HI, CH3 I, CF3 I and similar ones. A most suitable donor will be selected by these main criteria: high iodine yield, discharge stability, operation feasibility of donor, and also cost [Schmiedberger et. al., 2007]. Experimental setup A main part of the developed experimental device is shown in Fig. 1. A buffer gas (He, N2 ) is flowing from the channel (left side of the figure) into the cavity where an injector is located. This injector contains the discharge chamber with RF wire electrode and two channels for water cooling. The gaseous iodine compounds are introduced into the discharge chamber, where iodine atoms are generated by electric discharge and then injected through small holes into the buffer gas flow. The injector body has such a shape that its contour together with the wall forms a supersonic nozzle, where the gases will be mixed and cooled by expansion. Two types of RF supply are considered, a CW regime supply with frequency of 13.56 MHz and a pulsed regime supply with tunable frequency between 20 and 100 MHz (CW regime is also possible), both with power 25 - 500 W. Figure 1. Design of new discharge device for atomic iodine generation. From the left: duct of buffer gas, injector with RF discharge electrode and channel for water cooling. Injector together with the wall of the cavity forms the supersonic nozzle. Pitot tube is employed for pressure and flow velocity measurement. Final design of the device configuration, mainly the shape of the injector body was based on computational modeling, evaluating the flow properties (Mach number). For this task the CFD code Fluent was used. As a diagnostic tool we will use the optical emission spectroscopy, an optical spectrum analyzer Advantest Q 8384 will be used for this task. Its spectral range is from 600 to 1700 nm 195 PICKOVA ET AL.: DISCHARGE GENERATION OF ATOMIC IODINE (red and near infrared region) and maximum resolution 10 pm. Emitted light will be collected by optical fibre both from the discharge chamber and from the supersonic cavity outside the discharge region. Emission spectroscopy diagnostics Emission spectroscopy is the diagnostic technique widely used for measuring plasma parameters (temperature) and plasma composition i.e. species present in plasma like atoms, molecules and ions. A great advantage of emissive spectroscopy is that it is passive and therefore nondisturbing method i.e. does not change the state of plasma. According to measured wavelength we can employ the UV, optical, infrared and also other types of emission spectroscopy. It can be also divided to atomic and molecular spectroscopy. In our experiments we expect to have different atoms like e.g. I, H, F (depending on iodine donor used), atoms of buffer gas, and also different molecules: I2 , HI, H2 , CH3 , F2 , and some more. Atomic spectra are much simpler than molecular spectra due to a fact that molecule can rotate around its axes and distances between atoms in molecule can change (vibrations). On the other hand, there are many interactions also in atoms, so even theory of atomic spectra can be complicated [Martin et. al., 1996]. The theory of spectroscopy (mostly based on quantum mechanics) can be found in many books [Griem, 1964, Svanberg, 1992, Lochte-Holtgreven, 1968]. Tables with atomic and molecular spectra or spectral images can be also found in various Internet databases, such as HITRAN, NIST, VPL and JPL database (see References). Fig. 2. shows for example a part of the hydrogen iodide (HI) spectrum, which is of our interest. In databases only some of molecular spectra can be found, because many molecules have not been investigated yet. New data can be usually found in articles, for example in [Fantz, 2004]. Figure 2. Selection of hydrogen iodide (HI) spectral lines. Different sets of spectral lines forming molecular bands can be seen. Source: HITRAN database. To analyze the spectral correctly, we need to adopt some theory about the plasma behavior. Very important is usually the fact whether plasma is in thermodynamic equilibrium or at least local thermodynamic equilibrium (LTE). In thermodynamic equilibrium all the parts of the temperature of the whole system can be defined. LTE criteria can be adopted, when parts of the system are near the thermodynamic equilibrium and changes are very small, so temperature of each part can thus be defined and measured. Conditions for LTE are not always fulfilled, which is usually a problem of the low-pressure plasma characterized by a small amount of collisions. Conclusion We intend to continue in chemical oxygen-iodine laser and start the discharge oxygen-iodine laser research by employing RF discharge for atomic iodine generation. The effects of different modes of the discharge operation, different gas flows and also different iodine donors and buffer 196 PICKOVA ET AL.: DISCHARGE GENERATION OF ATOMIC IODINE gases on atomic iodine yield will be investigated. As a diagnostic tool we shall use optical emission spectroscopy to detect different emission lines of iodine, buffer gas, donor molecule and also different fragments and ions. Interpretation of these spectral measurements will be based on performing a detailed literature inspection of published tables or images of atomic and molecular spectra of species probably present in the discharge and also on spectroscopic theory. Chemical generation of both singlet oxygen and atomic iodine by chemical way will also continue in our laboratory. References Kodymová, J, O. Špalek, V. Jirásek, M. Čenský, Advances in the development of the oxygen-iodine laser, Czechoslovak Journal of Physics, 54, 561-574, 2004 Špalek, O., V. Jirásek, M. Čenský, J. Kodymová, I. Jakubec, G. D. Hager, Chemical generation of atomic iodine for chemical oxygen-iodine laser. II. Experimental results, Chemical physics, 282, 147-157, 2002. Jirásek, V., O. Špalek, J. Kodymová, M. Čenský, Chemical generation of atomic iodine for chemical oxygen-iodine laser. I. Modeling of reaction systems, Chemical physics, 269 , 167-178, 2001. Schmiedberger, J., V. Jirásek, J. Kodymová, K. Rohlena, Advanced Concept of Discharge Oxygen-Iodine Laser, AIAA Plasmadynamics and Lasers conference, 2007. Martin, W. C., W. L. Wiese, Atomic spectroscopy, A Compendium of Basic Ideas, Notation, Data and Formulas, National Institute of Standards and Technology (http://physics.nist.gov/Pubs/AtSpec/index.html), originally chapter 10 of the book of G. W. F. Drake (editor): Atomic, Molecular and Optical Physics Handbook, AIP Press, Woodbury, NY, 1996. Griem, H. R., Plasma Spectroscopy, McGraw-Hill, 1964 Svanberg, S., Atomic and molecular spectroscopy, Springer-Verlag, Berlin Heidelberg, 1992. Lochte-Holtgreven W., Plasma Diagnostics, North-Holland Publishing Company, Amsterdam, 1968. Fantz, U., Emission Spectroscopy of Molecular Low Pressure Plasmas, Contribution Plasma Physics, 44, 508-515, 2004. NIST database, http://www.nist.gov/ HITRAN database, http://www.cfa.harvard.edu/hitran/ VPL database, http://vpl.ipac.caltech edu/spectra/ JPL database, http://spec.jpl.nasa.gov 197