Spin Polarized Electron - Jordan University of Science and
... Spin polarized electron (SPE) beams have wide applications 1) Condensed matter 2) Atomic and molecular 3) High energy physics Historically, the development of a suitable SPE source and have been tried in attempts to produce beams of spin polarized electrons 1) Scattering from unpolarized target 2) P ...
... Spin polarized electron (SPE) beams have wide applications 1) Condensed matter 2) Atomic and molecular 3) High energy physics Historically, the development of a suitable SPE source and have been tried in attempts to produce beams of spin polarized electrons 1) Scattering from unpolarized target 2) P ...
electrons 2.notebook
... According to the Pauli exclusion principle, an atomic orbital may describe at most two electrons. To occupy the same orbital, two electrons must have opposite spins; that is, the electron spins must be paired. ...
... According to the Pauli exclusion principle, an atomic orbital may describe at most two electrons. To occupy the same orbital, two electrons must have opposite spins; that is, the electron spins must be paired. ...
The Hall Effect - Ryerson Department of Physics
... direction. This relationship can be used to determine the sign of the charge carriers present in any conducting material. It can be shown that for electrons moving in a semiconductor sample, the Hall voltage is equal to: ...
... direction. This relationship can be used to determine the sign of the charge carriers present in any conducting material. It can be shown that for electrons moving in a semiconductor sample, the Hall voltage is equal to: ...
Electromagnets Answers - Cockeysville Middle School
... of those. During a lecture in the year 1819, Hans Oersted had a compass sitting next to a wire. When Oersted completed the circuit by connecting the wire to a battery, the direction that the needle was pointing changed. This indicated that the electricity flowing through the wire had created a magne ...
... of those. During a lecture in the year 1819, Hans Oersted had a compass sitting next to a wire. When Oersted completed the circuit by connecting the wire to a battery, the direction that the needle was pointing changed. This indicated that the electricity flowing through the wire had created a magne ...
Q.M3 Home work 1 Due date 8.11.15 1
... 2)Find a state |Bi that is orthogonal to |Ai. Make sure |Bi is normalized. 3) Express |hi and |si in the {|Ai, |Bi} basis. 4) What are possible outcomes of a hardness measurement on the state |Ai, and with what probability will each occur? 5) Express the hardness operator in the {|Ai, |Bi} basis. ...
... 2)Find a state |Bi that is orthogonal to |Ai. Make sure |Bi is normalized. 3) Express |hi and |si in the {|Ai, |Bi} basis. 4) What are possible outcomes of a hardness measurement on the state |Ai, and with what probability will each occur? 5) Express the hardness operator in the {|Ai, |Bi} basis. ...
Electromagnetic Induction
... • The induced voltage in a coil is proportional to the product of the number of loops and the rate at which the magnetic field changes within those loops. • The amount of resulting current depends on the induced voltage but also on the resistance of the coil and the nature of the circuit (a property ...
... • The induced voltage in a coil is proportional to the product of the number of loops and the rate at which the magnetic field changes within those loops. • The amount of resulting current depends on the induced voltage but also on the resistance of the coil and the nature of the circuit (a property ...
quantum numbers - misshoughton.net
... Spin Quantum Number, ms needed to explain additional spectral line-splitting & different kinds of magnetism ferromagnetism-associated with substances containing Fe, Co & Ni paramagnetism-weak attraction to strong magnets (individual atoms vs. collection of atoms) paramagnetism couldn’t be ex ...
... Spin Quantum Number, ms needed to explain additional spectral line-splitting & different kinds of magnetism ferromagnetism-associated with substances containing Fe, Co & Ni paramagnetism-weak attraction to strong magnets (individual atoms vs. collection of atoms) paramagnetism couldn’t be ex ...
Concepts in Mesoscopic Physics
... The nature of decoherence can be complicated, involving various dynamic scattering mechanisms with different effectiveness that might depend on parameters such as temperature and magnetic field: lattice vibrations (electron-phonon scattering), scattering off the Coulomb potential created by other electr ...
... The nature of decoherence can be complicated, involving various dynamic scattering mechanisms with different effectiveness that might depend on parameters such as temperature and magnetic field: lattice vibrations (electron-phonon scattering), scattering off the Coulomb potential created by other electr ...
Lect14
... the +z direction, so initial potential energy is ZERO – This does NOT mean that the potential energy is a minimum!!! – When the loop is in the y-z plane and its magnetic moment points in the same direction as the field, its potential energy is NEGATIVE and is in fact the minimum. – Since U0 is not m ...
... the +z direction, so initial potential energy is ZERO – This does NOT mean that the potential energy is a minimum!!! – When the loop is in the y-z plane and its magnetic moment points in the same direction as the field, its potential energy is NEGATIVE and is in fact the minimum. – Since U0 is not m ...
Interacting electrons in a magnetic field: Mapping quantum
... It is important to realize that this mapping to a classical system transforms the FermiDirac statistics of the quantum-mechanical state at zero temperature to a classical Boltzmann system at temperature 1/(kB T ) = 2m. The Pauli principle, which hinders two electrons to occupy the same state, is enc ...
... It is important to realize that this mapping to a classical system transforms the FermiDirac statistics of the quantum-mechanical state at zero temperature to a classical Boltzmann system at temperature 1/(kB T ) = 2m. The Pauli principle, which hinders two electrons to occupy the same state, is enc ...
Shielding and Mitigations of the Magnetic Fields Generated by the
... shielding material, the highest shielding effectiveness is reached. U-typed screen displays are more effective than plain plate shaped. Accepted magnetic field limits should be revised, and in the light of recent research, regulations all over the world should be updated by reducing the general tren ...
... shielding material, the highest shielding effectiveness is reached. U-typed screen displays are more effective than plain plate shaped. Accepted magnetic field limits should be revised, and in the light of recent research, regulations all over the world should be updated by reducing the general tren ...
****** 1 - Weizmann Institute of Science
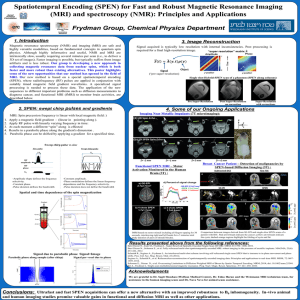
... 3D set of images). Faster imaging is possible, but typically suffers from image artifacts and is less robust. Our group is developing a new approach to collecting magnetic resonance data from nuclear spins, which is both faster and more robust than existing alternatives. This poster highlights some ...
... 3D set of images). Faster imaging is possible, but typically suffers from image artifacts and is less robust. Our group is developing a new approach to collecting magnetic resonance data from nuclear spins, which is both faster and more robust than existing alternatives. This poster highlights some ...
2008 midtermkey - University of Victoria
... A) Atomic orbitals describe regions in which an electron is most likely to be found around a nucleus. B) The three electrons in the configuration 2p3 have parallel spins (i.e. the same ms value). C) The fact that two electrons in the same atom cannot have the same set of four quantum numbers n, ℓ, m ...
... A) Atomic orbitals describe regions in which an electron is most likely to be found around a nucleus. B) The three electrons in the configuration 2p3 have parallel spins (i.e. the same ms value). C) The fact that two electrons in the same atom cannot have the same set of four quantum numbers n, ℓ, m ...
u2L1
... Electrostatics is the branch of Physics, which deals with the behavior of stationary electric charges. Charges are existing in two different kinds called positive and negative, these charges when in combination add algebraically i.e. the charge is a scalar quantity always quantized in integral mul ...
... Electrostatics is the branch of Physics, which deals with the behavior of stationary electric charges. Charges are existing in two different kinds called positive and negative, these charges when in combination add algebraically i.e. the charge is a scalar quantity always quantized in integral mul ...
The Modern Atomic Model
... • Showed electrons in controlled orbits. (like planets) – often called “Planetary Model”. • Experiments greater than 1 electron systems failed to reproduce this motion. ...
... • Showed electrons in controlled orbits. (like planets) – often called “Planetary Model”. • Experiments greater than 1 electron systems failed to reproduce this motion. ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.