QCD in strong magnetic field
... in our case) becomes effectively one-dimensional, because the particles tend to move along the magnetic field only. 2) Quarks interact stronger in one spatial dimension: In (1+1)D an arbitrarily weakest interaction between two objects leads to pair formation. This fact: (i) follows from Quantum Mech ...
... in our case) becomes effectively one-dimensional, because the particles tend to move along the magnetic field only. 2) Quarks interact stronger in one spatial dimension: In (1+1)D an arbitrarily weakest interaction between two objects leads to pair formation. This fact: (i) follows from Quantum Mech ...
Document
... Belief: Attractive force between the positively charged nucleus and an electron orbiting around is equal to the centrifugal exerted on the electron. This balance determines the electron’s radius. Challenge: A force is exerted on the electron, then, the electron should accelerate continuously accordi ...
... Belief: Attractive force between the positively charged nucleus and an electron orbiting around is equal to the centrifugal exerted on the electron. This balance determines the electron’s radius. Challenge: A force is exerted on the electron, then, the electron should accelerate continuously accordi ...
magnetic field - Lemon Bay High School
... A wire 36 m long carries a current of 22 A from east to west. If the magnetic force on the wire due to Earth’s magnetic field is downward (toward Earth) and has a magnitude of 4.0 10–2 N, find the magnitude and direction of the magnetic field at this ...
... A wire 36 m long carries a current of 22 A from east to west. If the magnetic force on the wire due to Earth’s magnetic field is downward (toward Earth) and has a magnitude of 4.0 10–2 N, find the magnitude and direction of the magnetic field at this ...
Kondo effect of an antidot in the integer quantum Hall regime: a
... PACS: 73.43.−f; 72.15.Qm Keywords: Antidot; Quantum Hall e ect; Kondo e ect ...
... PACS: 73.43.−f; 72.15.Qm Keywords: Antidot; Quantum Hall e ect; Kondo e ect ...
Resonant reflection at magnetic barriers in quantum wires - ITN
... can vanish if both a direct and an indirect transmission channel via the resonator are present and interfere with each other. This behavior corresponds to a Fano resonance,23 which is also found in systems related to ours.24,31,32 In the following, we argue that this scenario does exist in a magneti ...
... can vanish if both a direct and an indirect transmission channel via the resonator are present and interfere with each other. This behavior corresponds to a Fano resonance,23 which is also found in systems related to ours.24,31,32 In the following, we argue that this scenario does exist in a magneti ...
Avoided Antiferromagnetic Order and Quantum Critical Point in
... which have an antiferromagnetic (AFM) ground state at ambient pressure. The hydrostatic pressure suppresses the magnetic ordering temperature TN to zero at a critical pressure of the QCP. Such an approach was used successfully for CeCu2 Ge2 [8], CeRh2 Si2 [9], CePd2 Si2 and CeIn3 [4], and some other ...
... which have an antiferromagnetic (AFM) ground state at ambient pressure. The hydrostatic pressure suppresses the magnetic ordering temperature TN to zero at a critical pressure of the QCP. Such an approach was used successfully for CeCu2 Ge2 [8], CeRh2 Si2 [9], CePd2 Si2 and CeIn3 [4], and some other ...
Spin-Orbit Interaction - diss.fu
... the degeneracy of one-electron energy levels in atoms, molecules, and solids. In solid-state physics, the nonrelativistic Schrödinger equation is frequently used as a first approximation, e.g. in electron band-structure calculations. Without relativistic corrections, it leads to doubly-degenerated ...
... the degeneracy of one-electron energy levels in atoms, molecules, and solids. In solid-state physics, the nonrelativistic Schrödinger equation is frequently used as a first approximation, e.g. in electron band-structure calculations. Without relativistic corrections, it leads to doubly-degenerated ...
Topic 4: Materials - Education Umbrella
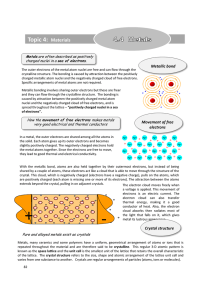
... With the metallic bond, atoms are also held together by their outermost electrons, but instead of being shared by a couple of atoms, these electrons act like a cloud that is able to move through the structure of the crystal. This cloud, which is negatively charged (electrons have a negative charge), ...
... With the metallic bond, atoms are also held together by their outermost electrons, but instead of being shared by a couple of atoms, these electrons act like a cloud that is able to move through the structure of the crystal. This cloud, which is negatively charged (electrons have a negative charge), ...
Summary
... to it and the applied magnetic field B. Each scattering event results in a recoiling atom (momentum =q) and a scattered photon (momentum =(k-q)). The recoiling atoms lie on a shell of radius =k. (B) Spontaneous Rayleigh scattering. The absorption image shows a halo of atoms. The intensity of the bea ...
... to it and the applied magnetic field B. Each scattering event results in a recoiling atom (momentum =q) and a scattered photon (momentum =(k-q)). The recoiling atoms lie on a shell of radius =k. (B) Spontaneous Rayleigh scattering. The absorption image shows a halo of atoms. The intensity of the bea ...
Period #2 Notes: Electronic Structure of Atoms
... • The field of particle physics and quantum mechanics is devoted to understanding electron orbitals, spins, and energy levels. ...
... • The field of particle physics and quantum mechanics is devoted to understanding electron orbitals, spins, and energy levels. ...
Study Guide: Chapter 4 - the Arrangement of Electrons in Atoms
... 2. Understand the relationship between energy of light and its frequency; Know how to calculate the energy of light given its frequency, and the frequency given its energy (MEMORIZE FORMULA; Planck’s constant will be given on the test) – work a few practice problems 3. Know what a line emission spec ...
... 2. Understand the relationship between energy of light and its frequency; Know how to calculate the energy of light given its frequency, and the frequency given its energy (MEMORIZE FORMULA; Planck’s constant will be given on the test) – work a few practice problems 3. Know what a line emission spec ...
Physics in Ultracold atoms
... How to reach ultracold temperature ? Normal pressure: He 4 4.2 K We need very low density to avoid strong binding, i.e. we need low “kinetic energy”, not low “potential energy” ! ...
... How to reach ultracold temperature ? Normal pressure: He 4 4.2 K We need very low density to avoid strong binding, i.e. we need low “kinetic energy”, not low “potential energy” ! ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.