- Post Graduate Government College
... The process is called spin-spin splitting • The splitting is into one more peak than the number of H’s on the adjacent carbon(s), This is the “n+1 rule” • The relative intensities are in proportion of a binomial distribution given by Pascal’s Triangle • The set of peaks is a multiplet (2 = doublet, ...
... The process is called spin-spin splitting • The splitting is into one more peak than the number of H’s on the adjacent carbon(s), This is the “n+1 rule” • The relative intensities are in proportion of a binomial distribution given by Pascal’s Triangle • The set of peaks is a multiplet (2 = doublet, ...
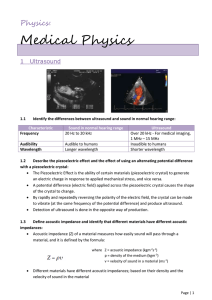
Medical Physics
... Describe how the principles of acoustic impedance and reflection and refraction are applied to ultrasound: A change in acoustic impedance is required for reflection to take place at a tissue boundary in the body. The produced ultrasound pulse travels through the skin of the patient until it reac ...
... Describe how the principles of acoustic impedance and reflection and refraction are applied to ultrasound: A change in acoustic impedance is required for reflection to take place at a tissue boundary in the body. The produced ultrasound pulse travels through the skin of the patient until it reac ...
Chapter 6:Electronic Structure of Atoms
... • Electron spin is crucial for the electron structure of atoms. • In 1925, Wolfgang Pauli discovered the principle that governs the arrangements of electrons in many electron atoms. • The Pauli Exclusion Principle states that no two electrons in an atom can have the same four quantum numbers, n, ʅ , ...
... • Electron spin is crucial for the electron structure of atoms. • In 1925, Wolfgang Pauli discovered the principle that governs the arrangements of electrons in many electron atoms. • The Pauli Exclusion Principle states that no two electrons in an atom can have the same four quantum numbers, n, ʅ , ...
Name
... The lines are field lines representing the magnetic field around the magnets. PTS: 1 DIF: L1 OBJ: 21.1.2 Interpret diagrams of magnetic field lines around one or more bar magnets. STA: SPS10.c.3 24. ANS: These lines are densest at the poles. PTS: 1 DIF: L2 OBJ: 21.1.3 Describe Earth’s magnetic field ...
... The lines are field lines representing the magnetic field around the magnets. PTS: 1 DIF: L1 OBJ: 21.1.2 Interpret diagrams of magnetic field lines around one or more bar magnets. STA: SPS10.c.3 24. ANS: These lines are densest at the poles. PTS: 1 DIF: L2 OBJ: 21.1.3 Describe Earth’s magnetic field ...
1 Early observations of and knowledge on air electricity and
... Peißenberg a deviation of 15 to 20 minutes was observed. After the end of the thunderstorm the magnetic needle came back to its former position.” Remarks in Ephemerides 1783 Duke de la Cepede is cited, who established as a result of relevant experiments, “that the many deviations of the magnetic ne ...
... Peißenberg a deviation of 15 to 20 minutes was observed. After the end of the thunderstorm the magnetic needle came back to its former position.” Remarks in Ephemerides 1783 Duke de la Cepede is cited, who established as a result of relevant experiments, “that the many deviations of the magnetic ne ...
Electrons
... Einstein – electromagnetic radiation (light) exhibits both wave and particle behavior. While light exhibits many wavelike properties, it can also be thought of as a stream of particles. ...
... Einstein – electromagnetic radiation (light) exhibits both wave and particle behavior. While light exhibits many wavelike properties, it can also be thought of as a stream of particles. ...
Magnetic Levitaion trains.pptx
... magnetic field of about 16 teslas at the Nijmegen High Field Magnet Laboratory. A substance which is diamagnetic repels a magnetic field. Earnshaw's theorem does not apply to diamagnets; they behave in the opposite manner of a typical magnet due to their relative permeability of μr < 1. All materia ...
... magnetic field of about 16 teslas at the Nijmegen High Field Magnet Laboratory. A substance which is diamagnetic repels a magnetic field. Earnshaw's theorem does not apply to diamagnets; they behave in the opposite manner of a typical magnet due to their relative permeability of μr < 1. All materia ...
Electron wavepackets and microscopic Ohm`s law (PPT
... • Each atomic state a band of states in the crystal These are the “allowed” states for electrons in the crystal Fill according to Pauli Exclusion Principle • There may be gaps between the bands These are “forbidden”energies where there are no states for electrons What do you expect to be a metal ...
... • Each atomic state a band of states in the crystal These are the “allowed” states for electrons in the crystal Fill according to Pauli Exclusion Principle • There may be gaps between the bands These are “forbidden”energies where there are no states for electrons What do you expect to be a metal ...
Magnetic nanoparticle traveling in external magnetic field
... It has been shown [5,6] for the first time that the conservation of the total momentum J leads to important consequences for the quantum tunneling of the magnetic moment of an isolated magnetic nanoparticle. However, the probability of quantum tunneling of the magnetic moment is nonzero [7] only for ...
... It has been shown [5,6] for the first time that the conservation of the total momentum J leads to important consequences for the quantum tunneling of the magnetic moment of an isolated magnetic nanoparticle. However, the probability of quantum tunneling of the magnetic moment is nonzero [7] only for ...
Ampere`s Law - Menihek Home Page
... The proportionality can be written as an equation when a constant of proportionality is inserted. Ampere was able to show that the constant was a very special one: it was μ !! Recall that μ is the magnetic permeability of the substance in which the field is located. The mathematical form of A ...
... The proportionality can be written as an equation when a constant of proportionality is inserted. Ampere was able to show that the constant was a very special one: it was μ !! Recall that μ is the magnetic permeability of the substance in which the field is located. The mathematical form of A ...
Effect of Magnetic Water and Phosphorous Rates on Growth and
... squash plant, in addition to their effect on some soil chemical properties. A randomized complete block design (RCBD) in factorial experiment with three replications was used. Summer squash (Mulla-Ahmed) seeds were sown in ridges 0.5 m apart and cultivated according to standard agriculture practices ...
... squash plant, in addition to their effect on some soil chemical properties. A randomized complete block design (RCBD) in factorial experiment with three replications was used. Summer squash (Mulla-Ahmed) seeds were sown in ridges 0.5 m apart and cultivated according to standard agriculture practices ...
Ionic crystals
... • Within each of the seven crystal systems one can find solids exhibiting the full range of electrical, mechanical, magnetic, superconducting, and optical ...
... • Within each of the seven crystal systems one can find solids exhibiting the full range of electrical, mechanical, magnetic, superconducting, and optical ...
s 1
... Includes He and Group II elements (e.g., Be, Mg, Ca, etc.). Valence electrons are indistinguishable, i.e., not physically possible to assign unique positions simultaneously. ...
... Includes He and Group II elements (e.g., Be, Mg, Ca, etc.). Valence electrons are indistinguishable, i.e., not physically possible to assign unique positions simultaneously. ...
Ferromagnetism

Not to be confused with Ferrimagnetism; for an overview see Magnetism.Ferromagnetism is the basic mechanism by which certain materials (such as iron) form permanent magnets, or are attracted to magnets. In physics, several different types of magnetism are distinguished. Ferromagnetism (including ferrimagnetism) is the strongest type: it is the only one that typically creates forces strong enough to be felt, and is responsible for the common phenomena of magnetism in magnets encountered in everyday life. Substances respond weakly to magnetic fields with three other types of magnetism, paramagnetism, diamagnetism, and antiferromagnetism, but the forces are usually so weak that they can only be detected by sensitive instruments in a laboratory. An everyday example of ferromagnetism is a refrigerator magnet used to hold notes on a refrigerator door. The attraction between a magnet and ferromagnetic material is ""the quality of magnetism first apparent to the ancient world, and to us today"".Permanent magnets (materials that can be magnetized by an external magnetic field and remain magnetized after the external field is removed) are either ferromagnetic or ferrimagnetic, as are other materials that are noticeably attracted to them. Only a few substances are ferromagnetic. The common ones are iron, nickel, cobalt and most of their alloys, some compounds of rare earth metals, and a few naturally-occurring minerals such as lodestone.Ferromagnetism is very important in industry and modern technology, and is the basis for many electrical and electromechanical devices such as electromagnets, electric motors, generators, transformers, and magnetic storage such as tape recorders, and hard disks.