Part 1 - Mechanics and Thermodynamics
... value. The smaller is the relative error the higher the accuracy of the measurement. When making operations with physical quantities, remember that the result may not be more accurate than the worst of the factors involved. For instance, when dividing 3.2165 m with 2.1 s to find the speed of some pa ...
... value. The smaller is the relative error the higher the accuracy of the measurement. When making operations with physical quantities, remember that the result may not be more accurate than the worst of the factors involved. For instance, when dividing 3.2165 m with 2.1 s to find the speed of some pa ...
Part V The Third Law and Free Energy
... produce further cooling by pumping away the vapor. The same is true for the magnetocaloric effect. For instance, if one demagnetization produces a temperature one-fifth of the original temperature, then a second demagnetization from the same original field will produce a temperature which is also ap ...
... produce further cooling by pumping away the vapor. The same is true for the magnetocaloric effect. For instance, if one demagnetization produces a temperature one-fifth of the original temperature, then a second demagnetization from the same original field will produce a temperature which is also ap ...
2.00atm x 1 .00L 0.0821 L.atm.mol K 298.15 = 8.17x10 mol. U = 8
... Example 1.1 The atmosphere consists of 78.08% by volume of N2, and 20.95% of O2. Calculate the partial pressures due to the two gases. The specification "% by volume" may be interpreted as follows. If the components of the atmosphere were to be separated, at the pressure of 1 atm, the volume occupie ...
... Example 1.1 The atmosphere consists of 78.08% by volume of N2, and 20.95% of O2. Calculate the partial pressures due to the two gases. The specification "% by volume" may be interpreted as follows. If the components of the atmosphere were to be separated, at the pressure of 1 atm, the volume occupie ...
MATHEMATICS OF COMPUTATION Volume 72, Number 241, Pages 131–157 S 0025-5718(01)01371-0
... 2). It has the advantage that the same strategy extends to multidimensions without any difficulties. The analysis of the properties of the schemes is performed in Section 3 and the strong convergence towards entropy solution is proved in Section 4. A variant of the scheme for more general cases of f ...
... 2). It has the advantage that the same strategy extends to multidimensions without any difficulties. The analysis of the properties of the schemes is performed in Section 3 and the strong convergence towards entropy solution is proved in Section 4. A variant of the scheme for more general cases of f ...
File
... ideal gases (no significant volume and little attraction for other molecules). The gases behave near ideal conditions until they are at: 1. extremely low temperatures 2. extremely high pressures For 1st year chemistry, we will assume that all gases ...
... ideal gases (no significant volume and little attraction for other molecules). The gases behave near ideal conditions until they are at: 1. extremely low temperatures 2. extremely high pressures For 1st year chemistry, we will assume that all gases ...
Fast Assembly of Gold Nanoparticles in Large
... methods for the fabrication of periodic nanostructures, self-assembly methods are particularly attractive because of their numerous benefits, including low costs and simple processing steps.7 Vertical8 and horizontal9 deposition of nanoparticles from a carrier solvent are established methods for the ...
... methods for the fabrication of periodic nanostructures, self-assembly methods are particularly attractive because of their numerous benefits, including low costs and simple processing steps.7 Vertical8 and horizontal9 deposition of nanoparticles from a carrier solvent are established methods for the ...
Investigating Freezing Point Depression and Cirrus Cloud
... Homogeneous freezing and melting experiments were performed using several different concentrations of aqueous ammonium sulfate particles. The results of the experiments are shown in Figure 1. In theory, when the aerosol particles are pure water (aw = 1), they should freeze at approximately 235 K.3 Th ...
... Homogeneous freezing and melting experiments were performed using several different concentrations of aqueous ammonium sulfate particles. The results of the experiments are shown in Figure 1. In theory, when the aerosol particles are pure water (aw = 1), they should freeze at approximately 235 K.3 Th ...
ch20powerpoint
... pairs, and justify your choice [assume constant temperature, except in part (e)]: (a) 1mol of SO2(g) or 1mol of SO3(g) (b) 1mol of CO2(s) or 1mol of CO2(g) (c) 3mol of oxygen gas (O2) or 2mol of ozone gas (O3) (d) 1mol of KBr(s) or 1mol of KBr(aq) (e) Seawater in midwinter at 20C or in midsummer at ...
... pairs, and justify your choice [assume constant temperature, except in part (e)]: (a) 1mol of SO2(g) or 1mol of SO3(g) (b) 1mol of CO2(s) or 1mol of CO2(g) (c) 3mol of oxygen gas (O2) or 2mol of ozone gas (O3) (d) 1mol of KBr(s) or 1mol of KBr(aq) (e) Seawater in midwinter at 20C or in midsummer at ...
week09.1.suspensions
... large surface area of dispersed particles results in high surface free energy DG = SL DA thermodynamically unstable can reduce SL by using surfactants but not often can one reach DG = 0 particles tend to come together ...
... large surface area of dispersed particles results in high surface free energy DG = SL DA thermodynamically unstable can reduce SL by using surfactants but not often can one reach DG = 0 particles tend to come together ...
Document
... is never in L1 (0, π/2) since (s + 1)/(s − 1) ∈ (1, 3). The singularity in ζ prevents us to define Q(f, f ) as a function unless f is smooth enough. To circumvent this difficulty, Arkeryd used the identity (11) as a definition of Q(f, f ). To make sense of this expression, no regularity is required ...
... is never in L1 (0, π/2) since (s + 1)/(s − 1) ∈ (1, 3). The singularity in ζ prevents us to define Q(f, f ) as a function unless f is smooth enough. To circumvent this difficulty, Arkeryd used the identity (11) as a definition of Q(f, f ). To make sense of this expression, no regularity is required ...
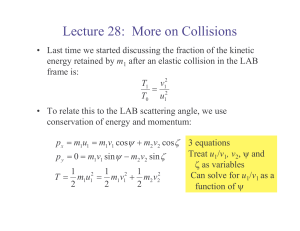
systems of particles
... • Although f ij and f ji are equal and opposite, the work of these forces will not, in general, cancel out. • If the forces acting on the particles are conservative, the work is equal to the change in potential energy and T1 V1 T2 V2 which expresses the principle of conservation of energy for ...
... • Although f ij and f ji are equal and opposite, the work of these forces will not, in general, cancel out. • If the forces acting on the particles are conservative, the work is equal to the change in potential energy and T1 V1 T2 V2 which expresses the principle of conservation of energy for ...
Engineering Analysis - Dynamics
... for reasonable accommodations. Contact the Office for Students with Disabilities, 321-433-5598, for eligibility criteria and more information; we recommend you do this within the first two weeks of class or preferably, before classes begin. Your expectation for confidentiality will be respected and ...
... for reasonable accommodations. Contact the Office for Students with Disabilities, 321-433-5598, for eligibility criteria and more information; we recommend you do this within the first two weeks of class or preferably, before classes begin. Your expectation for confidentiality will be respected and ...