Chemistry 180-213B Introductory Physical
... 2.1. Divertissement 1: Founders of the first law of thermodynamics If a tomb of the Unknown Scientists had been built in the 1850’s, the most appropriate inscription would have been "In memory of the grief and sacrifice of those who fought to realize a perpetuum mobile". But the law of conservation ...
... 2.1. Divertissement 1: Founders of the first law of thermodynamics If a tomb of the Unknown Scientists had been built in the 1850’s, the most appropriate inscription would have been "In memory of the grief and sacrifice of those who fought to realize a perpetuum mobile". But the law of conservation ...
A single parameter representation of hygroscopic
... activity expressions in Eq. (8), which can be seen by converting moles to volume and gathering all of the compositiondependent variables into the single parameter κ, here assumed not to vary with particle water content. However, no distinction is made between the soluble and insoluble portions of th ...
... activity expressions in Eq. (8), which can be seen by converting moles to volume and gathering all of the compositiondependent variables into the single parameter κ, here assumed not to vary with particle water content. However, no distinction is made between the soluble and insoluble portions of th ...
On the formation of radiation fogs under heavily polluted
... concentrations have been found in fogwater in California (Jacob et al., 1985), we are not trying to specifically model any observed pollution fogs, but rather to take a step forward from the simple Köhler theory calculations, in order to qualitatively find out which kind of effects soluble trace ga ...
... concentrations have been found in fogwater in California (Jacob et al., 1985), we are not trying to specifically model any observed pollution fogs, but rather to take a step forward from the simple Köhler theory calculations, in order to qualitatively find out which kind of effects soluble trace ga ...
Advanced Physical Chemistry Professor Angelo R. Rossi http
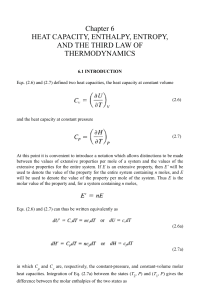
... The sequence of situations the system goes through in passing from the initial state to the final state is called the path taken by the system. Because the intensive variables often have no values during a process, it is usually not possible to exactly specify the path a process takes in terms of th ...
... The sequence of situations the system goes through in passing from the initial state to the final state is called the path taken by the system. Because the intensive variables often have no values during a process, it is usually not possible to exactly specify the path a process takes in terms of th ...
here
... The time rate of change of the linear momentum of a particle is equal to the net force acting on the particle This is the form in which Newton presented the Second Law It is a more general form than the one we used previously This form also allows for mass changes Momentum approach can be used ...
... The time rate of change of the linear momentum of a particle is equal to the net force acting on the particle This is the form in which Newton presented the Second Law It is a more general form than the one we used previously This form also allows for mass changes Momentum approach can be used ...
07-1 Note 07 Impulse and Momentum ∑ = ∑ =
... collision. Collisions form a class of problems in physics that involve two or more particles interacting in such a way that their states of motion are changed. We shall simplify our description by defining another kinematic quantity: linear momentum. 1 Isaac Newton called momentum an object’s “quant ...
... collision. Collisions form a class of problems in physics that involve two or more particles interacting in such a way that their states of motion are changed. We shall simplify our description by defining another kinematic quantity: linear momentum. 1 Isaac Newton called momentum an object’s “quant ...