Welcome to… Who Wants to be a Millionaire???
... Tell where the Maximum Potential Energy is and the Minimum Potential Energy is. ...
... Tell where the Maximum Potential Energy is and the Minimum Potential Energy is. ...
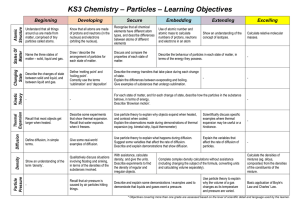
Atomic arrangement, short and long range order, point. Direction
... greatdistances the order becomes “blurred” and gradually gives way to “disorder.” which means that long-range order does not exist in liquids and amorphous solids. In crystals, the atoms are arranged in regular rows or networks (in threedimensional lattices), and aregular alternation of atoms separa ...
... greatdistances the order becomes “blurred” and gradually gives way to “disorder.” which means that long-range order does not exist in liquids and amorphous solids. In crystals, the atoms are arranged in regular rows or networks (in threedimensional lattices), and aregular alternation of atoms separa ...
NAME PERIOD ______ DATE MID-TERM STUDY GUIDE 6.0/HP
... B. She believed the sugar would make the flower grow faster. C. Shara filled 4 jars with 200 ml of water. Jar A had 10 ml of sugar added; Jar B had 20 ml. of sugar added and Jar C had 30 ml. of sugar added. D. Jar D had no sugar in it. E. The flowers in the sugar water all died. The more sugar in th ...
... B. She believed the sugar would make the flower grow faster. C. Shara filled 4 jars with 200 ml of water. Jar A had 10 ml of sugar added; Jar B had 20 ml. of sugar added and Jar C had 30 ml. of sugar added. D. Jar D had no sugar in it. E. The flowers in the sugar water all died. The more sugar in th ...
03 nanoparticles part 7 File - e-learning
... The technique results costly for those materials exhibiting low vapor pressure values (ceramics). In the other cases, the technique offers all advantages working in gas phase; moreover, it is possible to address the composition of the product by controlling adequately the composition of the atmosphe ...
... The technique results costly for those materials exhibiting low vapor pressure values (ceramics). In the other cases, the technique offers all advantages working in gas phase; moreover, it is possible to address the composition of the product by controlling adequately the composition of the atmosphe ...
2nd Semester Exam Review
... increase the surface area of a liquid by a given amount – Caused by intermolecular forces pulling down on the particles on the surface of a liquid which stretches it tight like a drum ...
... increase the surface area of a liquid by a given amount – Caused by intermolecular forces pulling down on the particles on the surface of a liquid which stretches it tight like a drum ...
NUCLEATION PRIMARY SECONDARY (induced by crystals
... Frequently nucleation can be promoted by agitation, shearing action, crystal breakage or abrasion, and pressure changes [Mul01]. There are different kinds of nucleation processes: If a solution contains no foreign particles or crystals of it’s own type, nucleus can only be formed by homogeneous nucl ...
... Frequently nucleation can be promoted by agitation, shearing action, crystal breakage or abrasion, and pressure changes [Mul01]. There are different kinds of nucleation processes: If a solution contains no foreign particles or crystals of it’s own type, nucleus can only be formed by homogeneous nucl ...
class notes packet - Social Circle City Schools
... Chromatography is a method for analyzing complex _______________ such as _____ by separating them into the chemicals fro which they are made. Attraction to a medium will take light particles up the medium as heavier particles stay low on the medium When can this be used? ...
... Chromatography is a method for analyzing complex _______________ such as _____ by separating them into the chemicals fro which they are made. Attraction to a medium will take light particles up the medium as heavier particles stay low on the medium When can this be used? ...
Word
... a. Water boils at 10 0C at 1 atm pressure b. Water boils above 100 0C at higher pressures c. Water boils below 100 0C at lower pressures C. Condensation 1. The conversion of a gas to a liquid by the removal of energy IV. Freezing and Melting A. Freezing Point 1. The temperature at which the solid an ...
... a. Water boils at 10 0C at 1 atm pressure b. Water boils above 100 0C at higher pressures c. Water boils below 100 0C at lower pressures C. Condensation 1. The conversion of a gas to a liquid by the removal of energy IV. Freezing and Melting A. Freezing Point 1. The temperature at which the solid an ...
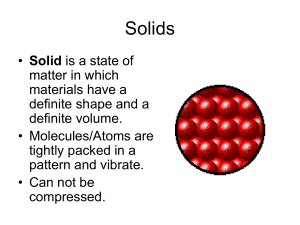
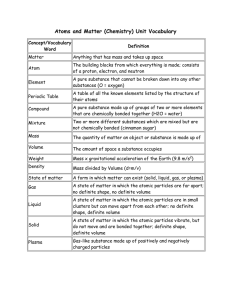
What are the four states of matter?
... Mass: amount of matter in an object Volume: amount of space an object occupies ...
... Mass: amount of matter in an object Volume: amount of space an object occupies ...
Chapter 3 PowerPoint Notes
... • Collisions between particles of a gas and the walls of the container cause the pressure in a closed container of gas. • Raising the temperature of a gas will increase its pressure if the volume and the number of particles are constant. • Reducing the volume of a gas will increase the pressure if t ...
... • Collisions between particles of a gas and the walls of the container cause the pressure in a closed container of gas. • Raising the temperature of a gas will increase its pressure if the volume and the number of particles are constant. • Reducing the volume of a gas will increase the pressure if t ...
File
... A state of matter in which the atomic particles are in small clusters but can move apart from each other; no definite ...
... A state of matter in which the atomic particles are in small clusters but can move apart from each other; no definite ...
FREEZING – is the change of a liquid to a solid. Freezing occurs
... Phase of matter is an important physical property of matter. Matter can exists in four phases: 1) Solid ...
... Phase of matter is an important physical property of matter. Matter can exists in four phases: 1) Solid ...
Changes of State
... The change of state from a liquid to a solid. The freezing point of a substance is the temperature at which a liquid changes into a solid. Freezing is the opposite process of melting. Freezing and melting occur at the same temperature. Freezing is an exothermic change because energy is taken out of ...
... The change of state from a liquid to a solid. The freezing point of a substance is the temperature at which a liquid changes into a solid. Freezing is the opposite process of melting. Freezing and melting occur at the same temperature. Freezing is an exothermic change because energy is taken out of ...
Crystal Growth
... Slow cooling of the melt • With congruently melting materials (those which maintain the same composition on melting), one simply melts a mixture of the desired composition then cools slowly (typically 2-10 °C/h) through the melting point. • More difficult with incongruently melting materials, knowl ...
... Slow cooling of the melt • With congruently melting materials (those which maintain the same composition on melting), one simply melts a mixture of the desired composition then cools slowly (typically 2-10 °C/h) through the melting point. • More difficult with incongruently melting materials, knowl ...
6 Departure from thermal equilibrium
... is the Planck mass. If the scattering process only involves massless particles, including in intermediate states, then by dimensional grounds it must go as Γ ∼ α2 T , where α = g 2 /4π and g is a typical coupling constant in the process. Thus in the very early universe, when T /Mp & α2 , this scatte ...
... is the Planck mass. If the scattering process only involves massless particles, including in intermediate states, then by dimensional grounds it must go as Γ ∼ α2 T , where α = g 2 /4π and g is a typical coupling constant in the process. Thus in the very early universe, when T /Mp & α2 , this scatte ...
Update on mechanical structure for the ENDCAP
... Measurement of the velocity of the propagation of the perturbation induced by the hammer along the longitudinal dimension of the crystal ...
... Measurement of the velocity of the propagation of the perturbation induced by the hammer along the longitudinal dimension of the crystal ...
View PDF
... etc. Aerosol particles consist of complex mixture of inorganic salts with hydrophilic and/or hygrophobic organic components which may evolved during their transportation into the atmosphere when they are exposed to gaseous traces and/or solar irradation. The properties of atmopsheric aerosols can be ...
... etc. Aerosol particles consist of complex mixture of inorganic salts with hydrophilic and/or hygrophobic organic components which may evolved during their transportation into the atmosphere when they are exposed to gaseous traces and/or solar irradation. The properties of atmopsheric aerosols can be ...