Ceramics for catalysis
... packed bed operation) or attrition (in reactors involving vigorous agitation). High surface areas can be attained either by fabricating small particles or clusters where the surface-to-volume ratio of each particle is high, or by creating materials where the void surface area (pores) is high compare ...
... packed bed operation) or attrition (in reactors involving vigorous agitation). High surface areas can be attained either by fabricating small particles or clusters where the surface-to-volume ratio of each particle is high, or by creating materials where the void surface area (pores) is high compare ...
Equilibrium (PowerPoint) West Coast 2015
... Students will understand and appreciate the relationship between kinetics and equilibrium. ...
... Students will understand and appreciate the relationship between kinetics and equilibrium. ...
Exam only.
... the enthalpy of reaction is the difference between product and reactant enthalpies. the Gibbs free energy is a function of both enthalpy and entropy. ...
... the enthalpy of reaction is the difference between product and reactant enthalpies. the Gibbs free energy is a function of both enthalpy and entropy. ...
Reaction rate and activation energy of the acidolysis
... 1.0 molar sodium hydroxide solution into a 1000 ml volumetric flask and filling up to the calibration mark with water. Fill the burette with 0.2 molar NaOH solution. Pipette 100 ml of 0.1 molar hydrochloric acid solution into an Erlenmeyer flask, seal it with a stopper, and temperature equilibrate i ...
... 1.0 molar sodium hydroxide solution into a 1000 ml volumetric flask and filling up to the calibration mark with water. Fill the burette with 0.2 molar NaOH solution. Pipette 100 ml of 0.1 molar hydrochloric acid solution into an Erlenmeyer flask, seal it with a stopper, and temperature equilibrate i ...
Wanganui High School
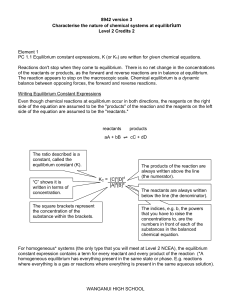
... o Increase in temperature favours the endothermic reaction o Decrease in temperature favours the exothermic reaction o Increase in conc. of a reactant (or decrease in concentration of a product) favours the formation of product / shifts equilibrium position to the right o Decrease in conc. of a reac ...
... o Increase in temperature favours the endothermic reaction o Decrease in temperature favours the exothermic reaction o Increase in conc. of a reactant (or decrease in concentration of a product) favours the formation of product / shifts equilibrium position to the right o Decrease in conc. of a reac ...
Equilibrium
... Indicator: Discuss why most chemical reactions do not proceed to completion Some chemical reactions proceed to completion: CH4 + O2 ---> CO2 + H2O Combustion reaction goes until all of the reactant is converted to product Most reactions do not go to completion: ...
... Indicator: Discuss why most chemical reactions do not proceed to completion Some chemical reactions proceed to completion: CH4 + O2 ---> CO2 + H2O Combustion reaction goes until all of the reactant is converted to product Most reactions do not go to completion: ...
1. A glucose molecule contains six carbons, twelve hydrogens and
... How much heat is required to warm the water in your 1.00 L water bottle from the temperature in your refrigerator (1.60 °C) to that in a typical classroom (23.0 °C)? Cs(H2O) = 4.18 J/g·°C, d(H2O) = 1.00 g/mL A. ...
... How much heat is required to warm the water in your 1.00 L water bottle from the temperature in your refrigerator (1.60 °C) to that in a typical classroom (23.0 °C)? Cs(H2O) = 4.18 J/g·°C, d(H2O) = 1.00 g/mL A. ...
Grade XII Foreign SET 2 Chemistry (Theory)
... In N2, the two nitrogen atoms form a triple bond. This triple bond has very high bond strength, which is very difficult to break. It is because of nitrogen’s small size that it is able to form p– p bonds with itself. This property is not exhibited by phosphorus. (ii)Sulphur hexafluoride (SF6) is k ...
... In N2, the two nitrogen atoms form a triple bond. This triple bond has very high bond strength, which is very difficult to break. It is because of nitrogen’s small size that it is able to form p– p bonds with itself. This property is not exhibited by phosphorus. (ii)Sulphur hexafluoride (SF6) is k ...
MSTA WOW Chemistry
... 1. Mix some water and food coloring in a plastic cup 2. If you lamp oil is green like the rubbing alcohol, then you need to add a different shade of food coloring to a small amount in a plastic cup. 3. Pour about 10 mL of Honey down the center of the graduated cylinder and try not to let it run down ...
... 1. Mix some water and food coloring in a plastic cup 2. If you lamp oil is green like the rubbing alcohol, then you need to add a different shade of food coloring to a small amount in a plastic cup. 3. Pour about 10 mL of Honey down the center of the graduated cylinder and try not to let it run down ...
General Equilibrium
... In dilute solutions, the activity coefficient approaches unity. Often, experimental conditions allow us to assume activity coefficients of one so that concentrations can be substituted for activities. (This assumption isn’t always good!) ...
... In dilute solutions, the activity coefficient approaches unity. Often, experimental conditions allow us to assume activity coefficients of one so that concentrations can be substituted for activities. (This assumption isn’t always good!) ...
Exam 1 Key
... K = PCO PH23 / PCH4 PH2O K = (0.18)(0.01)3 / (1)(1) = 1.8 × 10 -‐7 (b) What is ΔG° for this reaction at 600 K? (3 pts) ΔG° = -RT ln K = -(8.314 J mol-1 K-1)(600 K) ln 1.8 × 10-7 = 77.5 kJ mol-1 (c) Is the reaction endothermic or exothermic? Explain how you know. (3 pts) ΔG = ΔH - T ΔS where ΔG a ...
... K = PCO PH23 / PCH4 PH2O K = (0.18)(0.01)3 / (1)(1) = 1.8 × 10 -‐7 (b) What is ΔG° for this reaction at 600 K? (3 pts) ΔG° = -RT ln K = -(8.314 J mol-1 K-1)(600 K) ln 1.8 × 10-7 = 77.5 kJ mol-1 (c) Is the reaction endothermic or exothermic? Explain how you know. (3 pts) ΔG = ΔH - T ΔS where ΔG a ...
File
... At 150C the decomposition of acetaldehyde CH3CHO to methane is a first order reaction. If the rate constant for the reaction at 150C is 0.029 min-1, how long does it take a concentration of 0.050 mol L-1 of acetaldehyde to reduce to a concentration of 0.040 mol L-1? ...
... At 150C the decomposition of acetaldehyde CH3CHO to methane is a first order reaction. If the rate constant for the reaction at 150C is 0.029 min-1, how long does it take a concentration of 0.050 mol L-1 of acetaldehyde to reduce to a concentration of 0.040 mol L-1? ...
Question paper - Unit A173/02 - Module C7 - Higher tier
... whose work is used in this paper. To avoid the issue of disclosure of answer-related information to candidates, all copyright acknowledgements are reproduced in the OCR Copyright Acknowledgements Booklet. This is produced for each series of examinations and is freely available to download from our p ...
... whose work is used in this paper. To avoid the issue of disclosure of answer-related information to candidates, all copyright acknowledgements are reproduced in the OCR Copyright Acknowledgements Booklet. This is produced for each series of examinations and is freely available to download from our p ...
Chapter 17 - saddlespace.org
... (-) H XI. Reaction Rates Chemical kinetics: study of reaction rates and reaction mechanisms Reaction rate: the change in the concentration of REACTANTS per unit of time. Since the nature of reactant collisions determine how often reactions occur, changing the frequency and energy of these collisi ...
... (-) H XI. Reaction Rates Chemical kinetics: study of reaction rates and reaction mechanisms Reaction rate: the change in the concentration of REACTANTS per unit of time. Since the nature of reactant collisions determine how often reactions occur, changing the frequency and energy of these collisi ...
Rate of Reaction
... At some time, we observe that the reaction 2 N2O5 (g) → 4 NO2 (g) + O2 (g) is forming NO2 at the rate of 0.0072 mol / L∙s. (a) What is the rate of change of [O2], ∆ [O2]/ ∆t in mol / L∙s? (b) What is the rate of change of [N2O5], ∆ [N2O5]/ ∆t in mol / L∙s? ...
... At some time, we observe that the reaction 2 N2O5 (g) → 4 NO2 (g) + O2 (g) is forming NO2 at the rate of 0.0072 mol / L∙s. (a) What is the rate of change of [O2], ∆ [O2]/ ∆t in mol / L∙s? (b) What is the rate of change of [N2O5], ∆ [N2O5]/ ∆t in mol / L∙s? ...
Annexure `CD-01` L T P/S SW/FW TOTAL CREDIT UNITS 3 1 2 0 5
... This course gives and overall view on the various areas related to physical chemistry like chemical kinetics, colloidal state, adsorption etc which have great relevance in practical applications. The practical course is designed for imparting the knowledge of general principles of physical chemistry ...
... This course gives and overall view on the various areas related to physical chemistry like chemical kinetics, colloidal state, adsorption etc which have great relevance in practical applications. The practical course is designed for imparting the knowledge of general principles of physical chemistry ...
Equilibrium (Sheet 1)
... H2 + CO2 + heat. If no stress is introduced into this system, then the concentration of H 2O, CO, H2, and CO2 will not change. Now then, assume the concentration of H2O was increased, then effectively the number of collisions between H2O molecules and CO molecules are increased, resulting in an incr ...
... H2 + CO2 + heat. If no stress is introduced into this system, then the concentration of H 2O, CO, H2, and CO2 will not change. Now then, assume the concentration of H2O was increased, then effectively the number of collisions between H2O molecules and CO molecules are increased, resulting in an incr ...
EETopic Coversheet Word document
... which takes in heat (endothermic) for a reaction at equilibrium Know that an increase in concentration of reactants will favour the forward reaction (towards the products) for a reaction at equilibrium ...
... which takes in heat (endothermic) for a reaction at equilibrium Know that an increase in concentration of reactants will favour the forward reaction (towards the products) for a reaction at equilibrium ...
in a Chemical Reactor - Max-Planck
... benzene passes through many different reactors, mixing first with hydrogen and, afterwards, with oxygen. This last step is especially dangerous. The reaction carried out in the last step went out of control at the chemical plant in Flixborough, England, in 1974. The reactor exploded and 28 people di ...
... benzene passes through many different reactors, mixing first with hydrogen and, afterwards, with oxygen. This last step is especially dangerous. The reaction carried out in the last step went out of control at the chemical plant in Flixborough, England, in 1974. The reactor exploded and 28 people di ...
CHM222A: Basic Physical Chemistry
... Haber-Bosch Ammonia synthesis – illustrates thermodynamics, kinetics, gas transport processes, reaction dynamics, catalysis, surface processes Water phase diagram– illustrates phase equilibrium, molecular motions, solvation, electrolytic effects Kinesin molecular motors – illustrates irreversible pr ...
... Haber-Bosch Ammonia synthesis – illustrates thermodynamics, kinetics, gas transport processes, reaction dynamics, catalysis, surface processes Water phase diagram– illustrates phase equilibrium, molecular motions, solvation, electrolytic effects Kinesin molecular motors – illustrates irreversible pr ...
Document
... Reaction temperature 350-450 oC, Fe/Cr-catalyst, CO < 3 vol. % 3.b. Low-temperature conversion Reaction temperature 200-225 oC, Cu/Zn-catalyst, CO < 0.2 vol. % This reaction is carried out in two steps with intermediate heat removal. Initially, the process gas is passed through a bed of iron oxide/c ...
... Reaction temperature 350-450 oC, Fe/Cr-catalyst, CO < 3 vol. % 3.b. Low-temperature conversion Reaction temperature 200-225 oC, Cu/Zn-catalyst, CO < 0.2 vol. % This reaction is carried out in two steps with intermediate heat removal. Initially, the process gas is passed through a bed of iron oxide/c ...
The chemical master equation
... Boltzmann’s Stosszahlansatz (assumption of molecular chaos): Collisions cause a rapid loss of memory, i.e. particle trajectories can be treated as essentially random. Consequence: Chemical reactions can be treated as Markov (memoryless) processes, provided the Stosszahlansatz is satisfied. This in t ...
... Boltzmann’s Stosszahlansatz (assumption of molecular chaos): Collisions cause a rapid loss of memory, i.e. particle trajectories can be treated as essentially random. Consequence: Chemical reactions can be treated as Markov (memoryless) processes, provided the Stosszahlansatz is satisfied. This in t ...