Modeling the Rate of Heterogeneous Reactions
... simplified approach of the mean-field approximation for modeling technical catalytic reactors for many years. It is based on a continuum model, in which the surface of the catalyst is described as an array of equivalent sites which do interact neither before nor after chemisorption. Furthermore, the ...
... simplified approach of the mean-field approximation for modeling technical catalytic reactors for many years. It is based on a continuum model, in which the surface of the catalyst is described as an array of equivalent sites which do interact neither before nor after chemisorption. Furthermore, the ...
A Closure Study of the Reaction between Sulfur
... time led to little configuration changes. Nevertheless, four of these systems that contained two water molecules were followed for longer simulation runs, ca. 2000−2500 steps. They led to O3 evaporation from the cluster, whereas SO3− and SO3 remained in strong interaction with each other. Hence we co ...
... time led to little configuration changes. Nevertheless, four of these systems that contained two water molecules were followed for longer simulation runs, ca. 2000−2500 steps. They led to O3 evaporation from the cluster, whereas SO3− and SO3 remained in strong interaction with each other. Hence we co ...
AP Chemistry
... B. H+(aq) + Cl-(aq) + Na+ (aq) + OH- (aq) NaCl (aq) + H2O (l) C. Na+ (aq) + Cl- (aq) + Ag+ (aq) + NO3- (aq) AgCl (s) + Na+ (aq) + NO3- (aq) D. H+ (aq) + OH- (aq) H2O (l) E. CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) 1) Which of the reactions represents a net ionic equation? D (all others have spect ...
... B. H+(aq) + Cl-(aq) + Na+ (aq) + OH- (aq) NaCl (aq) + H2O (l) C. Na+ (aq) + Cl- (aq) + Ag+ (aq) + NO3- (aq) AgCl (s) + Na+ (aq) + NO3- (aq) D. H+ (aq) + OH- (aq) H2O (l) E. CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (l) 1) Which of the reactions represents a net ionic equation? D (all others have spect ...
One-Pot Catalytic Conversion of Cellulose and of Woody
... found for the latter substrate (Table 1). In each case, a H2, CO, CO2, CH4 gas mixture was generated while the liquid fraction contained HAE and CAE in a ratio consistent with cellulose/ hemicellulose and lignin origins, respectively. The reaction time required for solubilization of the biomass soli ...
... found for the latter substrate (Table 1). In each case, a H2, CO, CO2, CH4 gas mixture was generated while the liquid fraction contained HAE and CAE in a ratio consistent with cellulose/ hemicellulose and lignin origins, respectively. The reaction time required for solubilization of the biomass soli ...
Kinetics and Equilibrium ___ 1. In a chemical reaction the use of a
... ___ 1. In a chemical reaction the use of a catalyst results in a decrease in the (1) activation energy; (2) potential energy of the reactants; (3) heat of reaction; (4) amount of products. ___ 2. Given the reaction: CO2(s) <======> CO2(g). As the CO2(s) changes to CO 2(g) the entropy of the system ( ...
... ___ 1. In a chemical reaction the use of a catalyst results in a decrease in the (1) activation energy; (2) potential energy of the reactants; (3) heat of reaction; (4) amount of products. ___ 2. Given the reaction: CO2(s) <======> CO2(g). As the CO2(s) changes to CO 2(g) the entropy of the system ( ...
Chapter 11: Reactions of Alkyl Halides There are two basic types of
... The key to recognizing the inversion was noting that the optical activity was, although equal in size, now OPPOSITE in direction! The first reaction added something to the oxygen to make it leave. The third reaction chopped off something from the oxygen so it would return to normal. So it must be th ...
... The key to recognizing the inversion was noting that the optical activity was, although equal in size, now OPPOSITE in direction! The first reaction added something to the oxygen to make it leave. The third reaction chopped off something from the oxygen so it would return to normal. So it must be th ...
Electrochemical Preparation of Strong Bases Henning Lund
... with intermediate adsorbed hydrogen. Bases from acids with pKA-values up to 20 have been prepared by this method [2]. In organic chemistry even stronger bases are sometimes required for synthesis, and in such cases the anion of DMSO (“dimsyl”) may be employed. This reagent can be prepared by reactin ...
... with intermediate adsorbed hydrogen. Bases from acids with pKA-values up to 20 have been prepared by this method [2]. In organic chemistry even stronger bases are sometimes required for synthesis, and in such cases the anion of DMSO (“dimsyl”) may be employed. This reagent can be prepared by reactin ...
ppt - Wits Structural Chemistry
... Rate: • increases with ionic radius • decreases with an increase in ionic charge ...
... Rate: • increases with ionic radius • decreases with an increase in ionic charge ...
Advanced Chemistry
... 7) When the concentration of B in the reaction below is doubled, all other factors being held constant, it is found that the rate of the reaction remains unchanged. 2 A(g) + B(g) 2 C(g) The most probable explanation for this observation is that (A) The order of the reaction with respect to substa ...
... 7) When the concentration of B in the reaction below is doubled, all other factors being held constant, it is found that the rate of the reaction remains unchanged. 2 A(g) + B(g) 2 C(g) The most probable explanation for this observation is that (A) The order of the reaction with respect to substa ...
+ NO 2
... CHEMICAL REACTION (BONDS MUST BREAK) • SURFACE AREA/ CONTACT AREA (OPPORTUNITY FOR COLLISIONS) • CONCENTRATION ( INCREASE FREQUENCY) • TEMPERATURE ( INCREASE FREQUENCY) • CATALYST ( EFFECTIVE COLLISIONS) • NATURE OF REACTANTS ( EFFECTIVE COLLISIONS) ...
... CHEMICAL REACTION (BONDS MUST BREAK) • SURFACE AREA/ CONTACT AREA (OPPORTUNITY FOR COLLISIONS) • CONCENTRATION ( INCREASE FREQUENCY) • TEMPERATURE ( INCREASE FREQUENCY) • CATALYST ( EFFECTIVE COLLISIONS) • NATURE OF REACTANTS ( EFFECTIVE COLLISIONS) ...
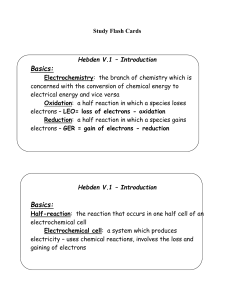
Hebden V.2 – Oxidation Numbers
... For titrations you need something that will change colour when it is oxidized – for an oxidizing agent use MnO4- for a reducing agent use ITo find the concentration of an element that is easily ...
... For titrations you need something that will change colour when it is oxidized – for an oxidizing agent use MnO4- for a reducing agent use ITo find the concentration of an element that is easily ...
Chapter 2 Kinetics of Chemical Reactions - diss.fu
... The chemical reactions of ions with molecules were intensively studied during the last century. It is assumed that this type of reactions plays an important role in the synthesis of large molecules inside interstellar clouds.30 Furthermore, the understanding of gas discharges and plasma also involve ...
... The chemical reactions of ions with molecules were intensively studied during the last century. It is assumed that this type of reactions plays an important role in the synthesis of large molecules inside interstellar clouds.30 Furthermore, the understanding of gas discharges and plasma also involve ...
Decomposition Reactions
... In order to better study and understand the incredible variety of possible chemical reactions, chemists classify reactions in a number of different ways. One common classification scheme recognizes four major types of chemical reactions: 1. combination or synthesis reactions 2. decomposition reactio ...
... In order to better study and understand the incredible variety of possible chemical reactions, chemists classify reactions in a number of different ways. One common classification scheme recognizes four major types of chemical reactions: 1. combination or synthesis reactions 2. decomposition reactio ...
Homogeneous and Heterogeneous Front Nucleation in a Bistable
... proceeds very slowly; Le., complete conversion takes place typically within 20-30 min. Themetastabilityhasgoneunnoticed in earlier experiments, since the beating rate has to be lowered drastically (from typically about 0.5 K/s to below 0.01 K/s) in order to make the hysteresis shrink markedly. The n ...
... proceeds very slowly; Le., complete conversion takes place typically within 20-30 min. Themetastabilityhasgoneunnoticed in earlier experiments, since the beating rate has to be lowered drastically (from typically about 0.5 K/s to below 0.01 K/s) in order to make the hysteresis shrink markedly. The n ...
Oxidation-Reduction Reactions
... oxidation number of +3. (Cu does not have oxidation numbers greater than +3.) Give only integer oxidation numbers. Answer: The oxidation numbers for Y, Ba, and O are +3, +2, and −2, respectively. Therefore, the sum of the oxidation numbers for the three Cu atoms must be +7. Two Cu atoms must have +2 ...
... oxidation number of +3. (Cu does not have oxidation numbers greater than +3.) Give only integer oxidation numbers. Answer: The oxidation numbers for Y, Ba, and O are +3, +2, and −2, respectively. Therefore, the sum of the oxidation numbers for the three Cu atoms must be +7. Two Cu atoms must have +2 ...
Final Exam - Seattle Central College
... – Indicate transition state, activation energy for reactants and products, ΔH for a reaction, effect of a catalyst. – Distinguish between the activated complex and the transition state. – Determine if a reaction is endothermic or exothermic. – Identify the number of steps in the mechanism for a cata ...
... – Indicate transition state, activation energy for reactants and products, ΔH for a reaction, effect of a catalyst. – Distinguish between the activated complex and the transition state. – Determine if a reaction is endothermic or exothermic. – Identify the number of steps in the mechanism for a cata ...
Chemical Kinetics
... isomerization, epimerization, and photolysis, and these processes may affect the stability of drugs in liquid, solid, and semisolid products. Mollica et al. reviewed the many effects that the ingredients of dosage forms and environmental factors may have on the chemical and physical stability of pha ...
... isomerization, epimerization, and photolysis, and these processes may affect the stability of drugs in liquid, solid, and semisolid products. Mollica et al. reviewed the many effects that the ingredients of dosage forms and environmental factors may have on the chemical and physical stability of pha ...
① Name AP CHEM __/__/__ Chapter 12 Outline
... example, living cells can only survive in a rather narrow temperature range, and the human body is designed to operate at an almost constant temperature of 98.6°F. But many of the complex biochemical reactions that take place at this temperature would be too slow at this temperature without interven ...
... example, living cells can only survive in a rather narrow temperature range, and the human body is designed to operate at an almost constant temperature of 98.6°F. But many of the complex biochemical reactions that take place at this temperature would be too slow at this temperature without interven ...
45. kinetics ch 12
... faster than one that is not because of a greater surface area for reactions. The concentrations of reactants: Most chemical reactions occur faster if the concentration of the reactants is increased. A higher concentration calls for more collisions, and more collisions lead to a faster reaction. The ...
... faster than one that is not because of a greater surface area for reactions. The concentrations of reactants: Most chemical reactions occur faster if the concentration of the reactants is increased. A higher concentration calls for more collisions, and more collisions lead to a faster reaction. The ...
Chemical reactions
... a direction in the process is implicit, i.e. initial reactants (left) converting into products (right), whereas the last form is more simple for equilibrium studies where no direction is privileged. Notice that microscopically the reaction is always on both directions, to the right and to the left i ...
... a direction in the process is implicit, i.e. initial reactants (left) converting into products (right), whereas the last form is more simple for equilibrium studies where no direction is privileged. Notice that microscopically the reaction is always on both directions, to the right and to the left i ...
simulating fritz haber`s ammonia synthesis with thermodynamic
... Lappeenranta University of Technology • 53851 Lappeenranta, Finland n 1909, Fritz Haber succeeded in synthesizing ammonia from hydrogen and atmospheric nitrogen. He was awarded the Nobel Prize in Chemistry in 1918. He presented the original experimental results in his Nobel lecture in 1920. This pap ...
... Lappeenranta University of Technology • 53851 Lappeenranta, Finland n 1909, Fritz Haber succeeded in synthesizing ammonia from hydrogen and atmospheric nitrogen. He was awarded the Nobel Prize in Chemistry in 1918. He presented the original experimental results in his Nobel lecture in 1920. This pap ...