A reaction - 固体表面物理化学国家重点实验室
... and one of the more popular methods for determining reaction order, is the half-life method. (半反应期) • The reaction half-life t1/2 is defined as the period of time necessary for the concentration of a specified reactant to reach one-half of its initial concentration. • Measurement of t1/2 as a functi ...
... and one of the more popular methods for determining reaction order, is the half-life method. (半反应期) • The reaction half-life t1/2 is defined as the period of time necessary for the concentration of a specified reactant to reach one-half of its initial concentration. • Measurement of t1/2 as a functi ...
Kinetics
... concentration) (units of concentration) Therefore : Units of rate constant= Units of rate/ (Units of concentration)2= M/sec/M2= M-1 S-1 Using Initial Rates to determine rate laws To determine the rate law, we observe the effect of changing the initial concentrations o If the reaction is zero ord ...
... concentration) (units of concentration) Therefore : Units of rate constant= Units of rate/ (Units of concentration)2= M/sec/M2= M-1 S-1 Using Initial Rates to determine rate laws To determine the rate law, we observe the effect of changing the initial concentrations o If the reaction is zero ord ...
Atmospheric Chemistry: CHEM-5151 / ATOC-5151
... at 10 km is determined by its reaction with OH. Jonathan scoffs and says that it is most definitely not so, and that it is the photolysis that controls PAN’s lifetime at that altitude. Their friend Karl (the only American in the group) offers up the slightly irrelevant piece of information that at t ...
... at 10 km is determined by its reaction with OH. Jonathan scoffs and says that it is most definitely not so, and that it is the photolysis that controls PAN’s lifetime at that altitude. Their friend Karl (the only American in the group) offers up the slightly irrelevant piece of information that at t ...
An Efficient Oxidation of Benzoins to Benzils by Manganese (II
... ligands generally afford air and moisture-stable complexes. On the other hand, coordination chemistry of manganese has been studied extensively so that that manganese center is surrounded by O- or N-donor ligands [13, 14]. In addition, they also act as catalysts for important reactions [15–20]. e de ...
... ligands generally afford air and moisture-stable complexes. On the other hand, coordination chemistry of manganese has been studied extensively so that that manganese center is surrounded by O- or N-donor ligands [13, 14]. In addition, they also act as catalysts for important reactions [15–20]. e de ...
Objectives - Dixie State University
... 8. Explain the difference between an exothermic and an endothermic reaction, including how their energy diagrams differ, whether their enthalpy is positive or negative, and what causes a reaction to be endothermic or exothermic. 9. Explain the relationship between G, H, and S. II. Kinetics of Reacti ...
... 8. Explain the difference between an exothermic and an endothermic reaction, including how their energy diagrams differ, whether their enthalpy is positive or negative, and what causes a reaction to be endothermic or exothermic. 9. Explain the relationship between G, H, and S. II. Kinetics of Reacti ...
Energetics of the primary electron transfer reaction revealed by
... reaction away from the special pair p is assumed to be monoexponential, even if the experiments on wild-type RCs and modified RCs R26.Phe-a have indicated some heterogeneity of this reaction step [ 12,131. Since interpretation of this feature does not interfere with the essence of this Letter, we do ...
... reaction away from the special pair p is assumed to be monoexponential, even if the experiments on wild-type RCs and modified RCs R26.Phe-a have indicated some heterogeneity of this reaction step [ 12,131. Since interpretation of this feature does not interfere with the essence of this Letter, we do ...
AP CHEMISTRY – Source: 1999 AP Exam, Also Data Base of MC
... (A) No further information is necessary. (B) Mass of solute (C) Mass of solute and mass of solvent (D) Mass of solute and volume of solvent (E) Mass of solute, mass of solvent, and vapor pressure of solvent 28. Which of the following is probably true for a solid solute with a highly endothermic heat ...
... (A) No further information is necessary. (B) Mass of solute (C) Mass of solute and mass of solvent (D) Mass of solute and volume of solvent (E) Mass of solute, mass of solvent, and vapor pressure of solvent 28. Which of the following is probably true for a solid solute with a highly endothermic heat ...
Briefing Session on 2012 HKDSE Examination (December 2012)
... ✔ It takes a long time to attain the equilibrium … ...
... ✔ It takes a long time to attain the equilibrium … ...
Unit 5 Notes
... standard state and are compared to the standard reduction potential of the hydrogen half-cell. ...
... standard state and are compared to the standard reduction potential of the hydrogen half-cell. ...
The Equilibrium Constant
... Number of molecules on the left side: __________. Number of molecules on the right side: __________. The pressure of the system would decrease if it shifted to the ________________. Why? ...
... Number of molecules on the left side: __________. Number of molecules on the right side: __________. The pressure of the system would decrease if it shifted to the ________________. Why? ...
Equilibrium Review True/False Indicate whether the statement is
... 5. (1 point) Changing the volume in the reaction vessel of an equilibrium system has an effect on _________. a. solids b. liquids c. gases d. all of the above e. none of the above ...
... 5. (1 point) Changing the volume in the reaction vessel of an equilibrium system has an effect on _________. a. solids b. liquids c. gases d. all of the above e. none of the above ...
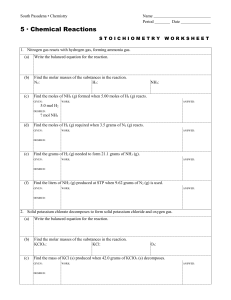
South Pasadena • Chemistry Name Period Date 5 · Chemical
... Find the volume of O2 (g) produced at STP when 42.0 grams of KClO3 (s) decomposes. GIVEN: ...
... Find the volume of O2 (g) produced at STP when 42.0 grams of KClO3 (s) decomposes. GIVEN: ...
Thermodynamics Free-Response
... The reaction above proceeds spontaneously from standard conditions at 298 K. (a) Predict the sign of the entropy change, ∆S◦, for the reaction. Explain. (b) How would the value of ∆S◦ for the reaction change if the product of the reaction was H2O (g)? (c) What is the sign of ∆G◦ at 298 K? Explain. ( ...
... The reaction above proceeds spontaneously from standard conditions at 298 K. (a) Predict the sign of the entropy change, ∆S◦, for the reaction. Explain. (b) How would the value of ∆S◦ for the reaction change if the product of the reaction was H2O (g)? (c) What is the sign of ∆G◦ at 298 K? Explain. ( ...
Wittig Reaction
... stereospecific alkenes from aldehydes or ketones reacting with phosphonium ylides. The Horner-WadsworthEmmons version performed in this experiment utilizes phosphonate esters as the ylide and specific in the formation of E-alkenes. Since toxic fumes may escape from the reaction setup, the procedure ...
... stereospecific alkenes from aldehydes or ketones reacting with phosphonium ylides. The Horner-WadsworthEmmons version performed in this experiment utilizes phosphonate esters as the ylide and specific in the formation of E-alkenes. Since toxic fumes may escape from the reaction setup, the procedure ...
First-Sample_Exam-1-Key
... Si(surface) + ½ H2(gas) Si-H(surface) The Si atoms are reacted with 4 mg of H2. (This reaction actually occurs during HF etching, and helps to protect the Si surface from oxide ...
... Si(surface) + ½ H2(gas) Si-H(surface) The Si atoms are reacted with 4 mg of H2. (This reaction actually occurs during HF etching, and helps to protect the Si surface from oxide ...
Examlette 1 - Bryn Mawr College
... 7. The standard free energy of formation for N2O4 is +97 kJ/mol and indicates that N2O4 is thermodynamically unstable towards decomposition to the elements. a) Explain what the term “standard free energy of formation “ means. Standard Free Energy refers to the energy required to form a molecule from ...
... 7. The standard free energy of formation for N2O4 is +97 kJ/mol and indicates that N2O4 is thermodynamically unstable towards decomposition to the elements. a) Explain what the term “standard free energy of formation “ means. Standard Free Energy refers to the energy required to form a molecule from ...
CO Oxidation on Palladium. 2. A Combined
... The temperature dependence of the COz formation rate on a Pd( 111) catalyst was studied in the temperature range of 470600Kusing a C 0 / 0 2 = 2/ 1reactant gas mixture a t a total pressure of 1.5 Torr. The total pressure change (APT)during the course of reaction is a very convenient and reliable way ...
... The temperature dependence of the COz formation rate on a Pd( 111) catalyst was studied in the temperature range of 470600Kusing a C 0 / 0 2 = 2/ 1reactant gas mixture a t a total pressure of 1.5 Torr. The total pressure change (APT)during the course of reaction is a very convenient and reliable way ...
Rate and Equilibrium
... the position of the equilibrium will shift in a direction that tends to reduce that change. Effect of concentration change on equilibrium: If the concentration of a substance involved in equilibrium is artificially increased, the reaction tends to adjust the composition so as to minimize the increas ...
... the position of the equilibrium will shift in a direction that tends to reduce that change. Effect of concentration change on equilibrium: If the concentration of a substance involved in equilibrium is artificially increased, the reaction tends to adjust the composition so as to minimize the increas ...
A Straightforward Route to Enantiopure Pyrrolizidines and
... together with methanol (MeOH) can be produced from syngas by appropriate modification of MeOHsynthesis catalysts and the reaction conditions [16-25]. Over the catalysts in this category, a significant amount of isobutanol is often produced. In the 1970s and 1980s, researchers at Union Carbide Corpor ...
... together with methanol (MeOH) can be produced from syngas by appropriate modification of MeOHsynthesis catalysts and the reaction conditions [16-25]. Over the catalysts in this category, a significant amount of isobutanol is often produced. In the 1970s and 1980s, researchers at Union Carbide Corpor ...
Equilibria PPT
... OCR’s resources are provided to support the teaching of OCR specifications, but in no way constitute an endorsed teaching method that is required by the Board, and the decision to use them lies with the individual teacher. Whilst every effort is made to ensure the accuracy of the content, OCR cannot ...
... OCR’s resources are provided to support the teaching of OCR specifications, but in no way constitute an endorsed teaching method that is required by the Board, and the decision to use them lies with the individual teacher. Whilst every effort is made to ensure the accuracy of the content, OCR cannot ...
Lecture 18. Chemical Equilibrium (Ch. 5)
... about the rate of the reaction. For this particular reaction, at the temperatures below 7000C, the rate is negligible (remember, the rapture of N-N and H-H bonds is an activation process). To increase the rate, either a high temperature or a good catalist is required. Haber Process, developed into a ...
... about the rate of the reaction. For this particular reaction, at the temperatures below 7000C, the rate is negligible (remember, the rapture of N-N and H-H bonds is an activation process). To increase the rate, either a high temperature or a good catalist is required. Haber Process, developed into a ...