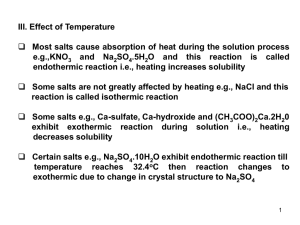
Molar Mass by Freezing Point Depression
... melting point without the formation of crystals - a phenomenon known as “supercooling”. As soon as the first crystals form, however, the temperature rises quickly to the melting point and remains constant. The heat (enthalpy) released by the solidification process exactly compensates the heat transf ...
... melting point without the formation of crystals - a phenomenon known as “supercooling”. As soon as the first crystals form, however, the temperature rises quickly to the melting point and remains constant. The heat (enthalpy) released by the solidification process exactly compensates the heat transf ...
Solutions
... substances that do not break up into ions (i.e. sugar, alcohols CxHyOH) Do not conduct an electric current (no mobile ions) Non-electrolytes have a dissociation factor (d.f.) of 1. C12H22O11 C12H22O11 (aq) 1 mole ...
... substances that do not break up into ions (i.e. sugar, alcohols CxHyOH) Do not conduct an electric current (no mobile ions) Non-electrolytes have a dissociation factor (d.f.) of 1. C12H22O11 C12H22O11 (aq) 1 mole ...
HCC9 Chapter 9 Objectives and Notes
... 1. law of definite proportions/law of constant composition: Discovered by Joseph Proust in the early 1800’s. In a given chemical compound the elements are always combined in the same proportion by mass. a. H2O is always 2.02 g hydrogen for every 16.0 g oxygen, the ratio of the masses never varies. 2 ...
... 1. law of definite proportions/law of constant composition: Discovered by Joseph Proust in the early 1800’s. In a given chemical compound the elements are always combined in the same proportion by mass. a. H2O is always 2.02 g hydrogen for every 16.0 g oxygen, the ratio of the masses never varies. 2 ...
Solubility
... • We can also determine the concentration of an ion necessary for precipitation to begin. • Assume that precipitation begins when Qsp = Ksp • Example: If a solution contains 0.0020 mol CrO42per liter, what concentration of Ag+ ion must be added as AgNO3 before Ag2CrO4 begins to precipitate. (Neglect ...
... • We can also determine the concentration of an ion necessary for precipitation to begin. • Assume that precipitation begins when Qsp = Ksp • Example: If a solution contains 0.0020 mol CrO42per liter, what concentration of Ag+ ion must be added as AgNO3 before Ag2CrO4 begins to precipitate. (Neglect ...
Lectures 5
... Increasing temperature of a dissolved gas leads to decreasing solubility of most gases due to greater tendency of the gas to expand at higher temperature. Therefore we should use caution when opening containers of gas at high temperature. e.g., ethyl nitrite vessel should be immersed in cold water o ...
... Increasing temperature of a dissolved gas leads to decreasing solubility of most gases due to greater tendency of the gas to expand at higher temperature. Therefore we should use caution when opening containers of gas at high temperature. e.g., ethyl nitrite vessel should be immersed in cold water o ...
Topic/Objective - cloudfront.net
... Objective: A small amount of a volatile liquid inside a small test tube and then heated in boiling water until all the liquid vaporizes and fills the tube as excess water escapes. After the gas is cooled, the mass, volume, and pressure is measured to determine the molecular mass of the substance usi ...
... Objective: A small amount of a volatile liquid inside a small test tube and then heated in boiling water until all the liquid vaporizes and fills the tube as excess water escapes. After the gas is cooled, the mass, volume, and pressure is measured to determine the molecular mass of the substance usi ...
Intermolecular and Ionic Forces
... (ice). This kit is specifically designed to show the difference between covalent bonds O-H bonds within a water molecule and the hydrogen bridges holding two different H2O molecules together. Groups will rotate through this station. The water molecule models must stay intact. After you are finished ...
... (ice). This kit is specifically designed to show the difference between covalent bonds O-H bonds within a water molecule and the hydrogen bridges holding two different H2O molecules together. Groups will rotate through this station. The water molecule models must stay intact. After you are finished ...
Current Density Distribution for a Full Scale Industrial Alluminization
... electrodeposition process from chloroaluminatebased ILs. In the field of FEA simulations, a typical aim is to obtain reliable models in order to “a priori” optimize the process setups [9]. We believe that this approach can be extended to electroplating industrial processes in order to determine the ...
... electrodeposition process from chloroaluminatebased ILs. In the field of FEA simulations, a typical aim is to obtain reliable models in order to “a priori” optimize the process setups [9]. We believe that this approach can be extended to electroplating industrial processes in order to determine the ...
Chemistry as a Game of Molecular Construction. The Bond-Click Way Brochure
... 8.2.6 Solubility and Insolubility of Ionic Materials 240 8.3 The Use of Ionic Matter in Living Organisms 242 8.3.1 Soluble Ionic Material Takes Care of Biological Communication 242 8.3.2 The Insoluble Ionic Material Makes Our Skeleton and Teeth 243 8.4 Covalent Molecules that Form Ions in Solution: ...
... 8.2.6 Solubility and Insolubility of Ionic Materials 240 8.3 The Use of Ionic Matter in Living Organisms 242 8.3.1 Soluble Ionic Material Takes Care of Biological Communication 242 8.3.2 The Insoluble Ionic Material Makes Our Skeleton and Teeth 243 8.4 Covalent Molecules that Form Ions in Solution: ...
Chemistry Chapter 4 Vocabulary 1. Solution 2. Solute 3. Solvent 4
... a substance that can donate a proton to another substance that has a ph below 7 a substance that can accept a proton from another substance that has a ph above 7 the concentration of hydrogen ions in a solution measurement of acidity describing a solution that is neither an acid nor a base substance ...
... a substance that can donate a proton to another substance that has a ph below 7 a substance that can accept a proton from another substance that has a ph above 7 the concentration of hydrogen ions in a solution measurement of acidity describing a solution that is neither an acid nor a base substance ...
Solutions. Electrolytic dissociation
... All solutes that dissolve in water can be divided in two categories: electrolytes and nonelectrolytes. An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. A nonelectrolyte solution does not conduct electricity. ...
... All solutes that dissolve in water can be divided in two categories: electrolytes and nonelectrolytes. An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. A nonelectrolyte solution does not conduct electricity. ...
72KB
... Sodium and chloride ions are held together by strong ionic bonds. A large amount of energy is required to overcome these forces. Hence NaCl has a high melting point. The forces of attraction between neighbouring water molecules in ice are weak. Only a small amount of energy is required to separate t ...
... Sodium and chloride ions are held together by strong ionic bonds. A large amount of energy is required to overcome these forces. Hence NaCl has a high melting point. The forces of attraction between neighbouring water molecules in ice are weak. Only a small amount of energy is required to separate t ...
Worksheet answers
... hydronium ion, H3O+. A proton (H+) cannot exist on its own in water! bases dissociate in water to form OH ions bases, such as NH3, that do not contain OH ions, produce OH by pulling H off water molecules acid + base salt + water titration - a solution’s concentration is determined by reacting i ...
... hydronium ion, H3O+. A proton (H+) cannot exist on its own in water! bases dissociate in water to form OH ions bases, such as NH3, that do not contain OH ions, produce OH by pulling H off water molecules acid + base salt + water titration - a solution’s concentration is determined by reacting i ...
Solubility Product Constants We have been looking at how
... In the equilibrium expression for the dissolving of an ionic solid there are actually two constants, the Keq and the concentration of the solid. If you change the number of moles of solid, the volume of the solid changes proportionally, so the concentration is a constant. Chemists leave out the conc ...
... In the equilibrium expression for the dissolving of an ionic solid there are actually two constants, the Keq and the concentration of the solid. If you change the number of moles of solid, the volume of the solid changes proportionally, so the concentration is a constant. Chemists leave out the conc ...
JF CH 1101 General and Physical Chemistry 2013
... Ionic solutions are characterised by their conductivity . Recall from basic physics that the resistance R of a material sample depends on the geometry of the sample. R is directly proportional to the length L of the sample, and inversely proportional to the cross sectional area A of the sample. We ...
... Ionic solutions are characterised by their conductivity . Recall from basic physics that the resistance R of a material sample depends on the geometry of the sample. R is directly proportional to the length L of the sample, and inversely proportional to the cross sectional area A of the sample. We ...
Solution-Solubility-Equilibrium
... Some substances dissolve in each other in all proportions. Examples are water and antifreeze, or gasoline and kerosene. Such substances are said to be miscible. Most other substances are partially soluble, that is, one dissolves in the other to a concentration that reaches a definite limiting value ...
... Some substances dissolve in each other in all proportions. Examples are water and antifreeze, or gasoline and kerosene. Such substances are said to be miscible. Most other substances are partially soluble, that is, one dissolves in the other to a concentration that reaches a definite limiting value ...
Lecture 19 - University of Windsor
... (2) b2 6 b3 Solid Na deposits, but reaction happens to make Na2K (K atoms diffuse into solid Na) Here, liquid Na/K in eqb. with Na2K solid (3) b3 6 b4 Amount of solid increases until b4, liquid hits eutectic point, now a two phase solid is formed Incongruent melting point: The temperature at which o ...
... (2) b2 6 b3 Solid Na deposits, but reaction happens to make Na2K (K atoms diffuse into solid Na) Here, liquid Na/K in eqb. with Na2K solid (3) b3 6 b4 Amount of solid increases until b4, liquid hits eutectic point, now a two phase solid is formed Incongruent melting point: The temperature at which o ...
K b
... Buffer pKa depends on temperature. Tris has an exceptionally large dependence, −0.028 pKa units per degree, near room temperature. A solution of tris with pH 8.07 at 25°C will have pH ≈ 8.7 at 4°C and pH ≈ 7.7 at 37°C. Changing temperature changes pH. ...
... Buffer pKa depends on temperature. Tris has an exceptionally large dependence, −0.028 pKa units per degree, near room temperature. A solution of tris with pH 8.07 at 25°C will have pH ≈ 8.7 at 4°C and pH ≈ 7.7 at 37°C. Changing temperature changes pH. ...
Solutions, Solubility Rules, and Molarity File
... • A nonelectrolyte may dissolve in water, but it does not dissociate into ions when it does so. – Solutions do not conduct electricity ...
... • A nonelectrolyte may dissolve in water, but it does not dissociate into ions when it does so. – Solutions do not conduct electricity ...
Concentration of solutions
... • properties that depend on the concentration of solute particles but not on their identity. • Vapor pressure lowering. Boiling point is higher and freezing point of a solution is lower than that of a pure solvent. This is due to the presence of nonvolatile solutes. These are substances that have li ...
... • properties that depend on the concentration of solute particles but not on their identity. • Vapor pressure lowering. Boiling point is higher and freezing point of a solution is lower than that of a pure solvent. This is due to the presence of nonvolatile solutes. These are substances that have li ...
Solutions - WordPress.com
... • The most common solvent is Water – the universal solvent • The term aqueous or (aq) describes a solution in which the solvent is water • Not all solutes and solvents are liquid! ▫ Brass (zinc and nickel) ▫ Seawater (salt and other substances in water) ▫ Air (Many different gasses in Nitrogen) ...
... • The most common solvent is Water – the universal solvent • The term aqueous or (aq) describes a solution in which the solvent is water • Not all solutes and solvents are liquid! ▫ Brass (zinc and nickel) ▫ Seawater (salt and other substances in water) ▫ Air (Many different gasses in Nitrogen) ...
Class Syllabus Ch120a: Nature of the Chemical Bond Units: 3-0
... engineering, applied physics, biochemistry, physics, geophysics, and mechanical engineering with an interest in characterizing and designing molecules, drugs, and materials. ...
... engineering, applied physics, biochemistry, physics, geophysics, and mechanical engineering with an interest in characterizing and designing molecules, drugs, and materials. ...
Class Syllabus
... and theorists in chemistry, materials science, chemical engineering, applied physics, biochemistry, physics, geophysics, and mechanical engineering with an interest in characterizing and designing molecules, drugs, and materials. Courses in QM often focus more on applied mathematics rather than phys ...
... and theorists in chemistry, materials science, chemical engineering, applied physics, biochemistry, physics, geophysics, and mechanical engineering with an interest in characterizing and designing molecules, drugs, and materials. Courses in QM often focus more on applied mathematics rather than phys ...
Switchable silver mirrors with long memory effects
... lm, to achieve a mirror state, and the dissolution of Ag as an ion into an electrolyte, to achieve a transparent state. While the transparent state is quite stable, the mirror state is unstable because the deposited Ag lm is dissolved into the electrolyte solution as anions diffuse into the metalli ...
... lm, to achieve a mirror state, and the dissolution of Ag as an ion into an electrolyte, to achieve a transparent state. While the transparent state is quite stable, the mirror state is unstable because the deposited Ag lm is dissolved into the electrolyte solution as anions diffuse into the metalli ...
Ionic liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions and short-lived ion pairs. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many applications, such as powerful solvents and electrically conducting fluids (electrolytes). Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been used as sealants due to their very low vapor pressure.Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at 801 °C (1,474 °F) into a liquid that consists largely of sodium cations (Na+) and chloride anions (Cl−). Conversely, when an ionic liquid is cooled, it often forms an ionic solid—which may be either crystalline or glassy.The ionic bond is usually stronger than the Van der Waals forces between the molecules of ordinary liquids. For that reason, common salts tend to melt at higher temperatures than other solid molecules. Some salts are liquid at or below room temperature. Examples include compounds based on the 1-Ethyl-3-methylimidazolium (EMIM) cation and include: EMIM:Cl, EMIM dicyanamide, (C2H5)(CH3)C3H3N+2·N(CN)−2, that melts at −21 °C (−6 °F); and 1-butyl-3,5-dimethylpyridinium bromide which becomes a glass below −24 °C (−11 °F).Low-temperature ionic liquids can be compared to ionic solutions, liquids that contain both ions and neutral molecules, and in particular to the so-called deep eutectic solvents, mixtures of ionic and non-ionic solid substances which have much lower melting points than the pure compounds. Certain mixtures of nitrate salts can have melting points below 100 °C.The term ""ionic liquid"" in the general sense was used as early as 1943.