Identification - KHAZAR UNIVERSITY
... between solids, liquids and gases (90) Spontaneity and Equilibrium (203220) The general conditions of equilibrium and for spontaneity (203) Conditions for equilibrium and spontaneity under constraints (204) Driving forces for natural changes (208) The fundamental equations of thermodynamics (208) Th ...
... between solids, liquids and gases (90) Spontaneity and Equilibrium (203220) The general conditions of equilibrium and for spontaneity (203) Conditions for equilibrium and spontaneity under constraints (204) Driving forces for natural changes (208) The fundamental equations of thermodynamics (208) Th ...
4 hon chem classifying matter b
... Depend on how much (the extent) matter there is i.e. length, mass, volume ...
... Depend on how much (the extent) matter there is i.e. length, mass, volume ...
File - Flipped Out Science with Mrs. Thomas!
... reaction. When a state of matter change occurs, it is usually because there is an increase or decrease of heat. Think about a chocolate bunny. If you leave it out in the sun, it melts. But if you had it on a plate, you could, in theory, put the plate of melted chocolate in the fridge and it would fo ...
... reaction. When a state of matter change occurs, it is usually because there is an increase or decrease of heat. Think about a chocolate bunny. If you leave it out in the sun, it melts. But if you had it on a plate, you could, in theory, put the plate of melted chocolate in the fridge and it would fo ...
5 - BrainMass
... 298.15 K (25°C), calculate the value of ΔH° for each of the following reactions: a. N2O4 (g) + 4 H2 (g) N2 (g) + 4 H2O (g) b. 2 KOH(s) + CO2 (g) K2CO3(s) + H2O (g) c. SO2 (g) + 2 H2S (g) 3/8 S8(s) + 2 H2O (g) d. Fe2O3(s) + 6 HCl (g) 2 FeCl3(s) + 3 H2O (g) 5.114) A sample of a hydrocarbon is ...
... 298.15 K (25°C), calculate the value of ΔH° for each of the following reactions: a. N2O4 (g) + 4 H2 (g) N2 (g) + 4 H2O (g) b. 2 KOH(s) + CO2 (g) K2CO3(s) + H2O (g) c. SO2 (g) + 2 H2S (g) 3/8 S8(s) + 2 H2O (g) d. Fe2O3(s) + 6 HCl (g) 2 FeCl3(s) + 3 H2O (g) 5.114) A sample of a hydrocarbon is ...
Chapter 2 Matter and Change
... *physical - composition of matter never changes *chemical – composition of matter always changes ...
... *physical - composition of matter never changes *chemical – composition of matter always changes ...
COURSE SYLLABUS CHEM 433 – Physical Chemistry I Fall 2014
... Dr. Sudeep Bhattacharyay P-319, MTWR (11:00 – 11:50 AM) P-455 ...
... Dr. Sudeep Bhattacharyay P-319, MTWR (11:00 – 11:50 AM) P-455 ...
RTF
... False. Because the rate of the forward reaction equals the rate of the reverse reaction, the concentrations of the reactants decreases (as the forward reaction occurs) at the same rate that the concentration of the reactants increases (as the reverse reaction occurs). There is no net change in react ...
... False. Because the rate of the forward reaction equals the rate of the reverse reaction, the concentrations of the reactants decreases (as the forward reaction occurs) at the same rate that the concentration of the reactants increases (as the reverse reaction occurs). There is no net change in react ...
AP Chemistry Syllabus 2013 Mawhiney
... 5. Use the properties of metals and nonmetals to predict reaction products. 6. Write chemical equations for synthesis, decomposition, single replacement, metathetical, redox, combustion, and acid-base reactions. 7. Use the Periodic Table to predict common oxidation states. 8. Use the Activity series ...
... 5. Use the properties of metals and nonmetals to predict reaction products. 6. Write chemical equations for synthesis, decomposition, single replacement, metathetical, redox, combustion, and acid-base reactions. 7. Use the Periodic Table to predict common oxidation states. 8. Use the Activity series ...
Atoms, Elements, Compounds File
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
... SOL 6.4 Atoms, Elements, compounds The student will investigate and understand that all matter is made up of atoms. Key concepts include ...
Lab Science 9 Pacing Guide
... 8. Use historical examples to explain how new ideas are limited by the context in which they are conceived; are often initially rejected by the scientific establishment; sometimes spring from unexpected findings; and usually grow slowly through contributions from many different investigators (e.g., ...
... 8. Use historical examples to explain how new ideas are limited by the context in which they are conceived; are often initially rejected by the scientific establishment; sometimes spring from unexpected findings; and usually grow slowly through contributions from many different investigators (e.g., ...
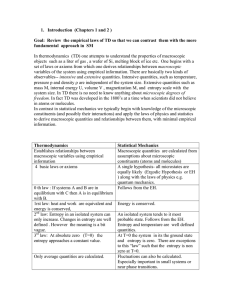
1. Introduction (Chapters 1 and 2 ) Goal: Review the empirical laws
... 1. Introduction (Chapters 1 and 2 ) Goal: Review the empirical laws of TD so that we can contrast them with the more fundamental approach in SM In thermodynamics (TD) one attempts to understand the properties of macroscopic objects such as a liter of gas , a wafer of Si, melting block of ice etc. On ...
... 1. Introduction (Chapters 1 and 2 ) Goal: Review the empirical laws of TD so that we can contrast them with the more fundamental approach in SM In thermodynamics (TD) one attempts to understand the properties of macroscopic objects such as a liter of gas , a wafer of Si, melting block of ice etc. On ...
Types of Chemical Reactions - Celebrity Examples
... y Emission of heat and y Giving off light y Formation of a precipitate y Formation of a gas y Color change ...
... y Emission of heat and y Giving off light y Formation of a precipitate y Formation of a gas y Color change ...
Types of Chemical Reactions
... of a combination of carbon and hydrogen) to form water and carbon dioxide. These reactions are exothermic, meaning they produce heat. This reaction can be expressed as: ...
... of a combination of carbon and hydrogen) to form water and carbon dioxide. These reactions are exothermic, meaning they produce heat. This reaction can be expressed as: ...
Balancing Equations
... • I can list the main types of chemical reactions. • I can identify reactants and products in a chemical equation. • I can balance chemical equations when all reactants and products are given. • I can classify reactions by major type and can predict products of simple reactions. • I can apply the st ...
... • I can list the main types of chemical reactions. • I can identify reactants and products in a chemical equation. • I can balance chemical equations when all reactants and products are given. • I can classify reactions by major type and can predict products of simple reactions. • I can apply the st ...
chemical equation - Central Lyon CSD
... numbers are called coefficients—small whole numbers that are placed in front of the formulas in an equation in order to balance it. ...
... numbers are called coefficients—small whole numbers that are placed in front of the formulas in an equation in order to balance it. ...
Thermodynamics Notes
... An irreversible equilibrium cannot be reversed without the help of an external agency and without changing the properties of the surroundings. Cyclic process A process in which the system undergoes a series of changes and ultimately returns to its original state. ...
... An irreversible equilibrium cannot be reversed without the help of an external agency and without changing the properties of the surroundings. Cyclic process A process in which the system undergoes a series of changes and ultimately returns to its original state. ...
Erik`s Chemistry: Thermochemistry - ECHS Chemistry
... ! In some textbooks H is written as a product or reactant ! The preceding is based upon the Law of Conservation of Energy (James Joule, 1818-1889, Joule also developed the First Law of Thermodynamics): energy is neither created nor destroyed in ordinary chemical or physical changes. b. Quantitative ...
... ! In some textbooks H is written as a product or reactant ! The preceding is based upon the Law of Conservation of Energy (James Joule, 1818-1889, Joule also developed the First Law of Thermodynamics): energy is neither created nor destroyed in ordinary chemical or physical changes. b. Quantitative ...
Inside the Atom connections to the lower secondary (KS3
... • a simple (Dalton) atomic model • differences between atoms, elements and compounds • chemical symbols and formulae for elements and compounds • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curriculum is taught in the chemi ...
... • a simple (Dalton) atomic model • differences between atoms, elements and compounds • chemical symbols and formulae for elements and compounds • conservation of mass changes of state and chemical reactions. Most of the nuclear physics related content in the KS3 curriculum is taught in the chemi ...
Ch17-2 Driving Forces of Reactions
... 5. catalyst (only effects rate does not participate in reaction…can recover.) ...
... 5. catalyst (only effects rate does not participate in reaction…can recover.) ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.