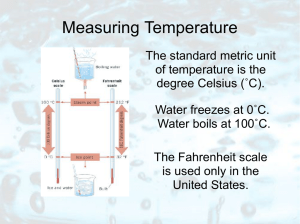
Measuring Temperature
... stated as the Law of Entropy: The total entropy (or microscopic disorganization) of the participants in any physical process cannot decrease during that process, but it can increase. That's not to say that you can't create a little more order in some part of your world, but the price you pay is to c ...
... stated as the Law of Entropy: The total entropy (or microscopic disorganization) of the participants in any physical process cannot decrease during that process, but it can increase. That's not to say that you can't create a little more order in some part of your world, but the price you pay is to c ...
Thermochemistry ppt with inkings
... State Functions • State function: depends only on the initial and final states of system, not on how the internal energy is used. ...
... State Functions • State function: depends only on the initial and final states of system, not on how the internal energy is used. ...
Physical or Chemical Properties
... Physical Change With a physical change no new substance is created and the original matter can be recovered. Physical change does not change the composition of the matter. The original matter is still present. The substance may seem different, but the way the atoms are linked up are the same. ...
... Physical Change With a physical change no new substance is created and the original matter can be recovered. Physical change does not change the composition of the matter. The original matter is still present. The substance may seem different, but the way the atoms are linked up are the same. ...
quarter 4 final exam guide - District 196 e
... and cooled to 20.0 degrees Celcius. How much energy is given off in this entire process? (Use a diagram “temperature versus time” to visualize what happens as energy is removed from the system – identify the potential and kinetic energy changes on this diagram.) ...
... and cooled to 20.0 degrees Celcius. How much energy is given off in this entire process? (Use a diagram “temperature versus time” to visualize what happens as energy is removed from the system – identify the potential and kinetic energy changes on this diagram.) ...
Physical or Chemical Properties
... Physical Change With a physical change no new substance is created and the original matter can be recovered. Physical change does not change the composition of the matter. The original matter is still present. The substance may seem different, but the way the atoms are linked up are the same. ...
... Physical Change With a physical change no new substance is created and the original matter can be recovered. Physical change does not change the composition of the matter. The original matter is still present. The substance may seem different, but the way the atoms are linked up are the same. ...
Ppt19(PS8)_Thermo_Hess
... What is the final temperature in a squeezed cold pack that contains 50.0 g of NH4NO3 dissolved in 125 mL of water? Assume the specific heat capacity of the dissolved NH4NO3 is negligible compared to water, an initial temperature of 25.0 C, and no heat transfer between the cold pack and the environm ...
... What is the final temperature in a squeezed cold pack that contains 50.0 g of NH4NO3 dissolved in 125 mL of water? Assume the specific heat capacity of the dissolved NH4NO3 is negligible compared to water, an initial temperature of 25.0 C, and no heat transfer between the cold pack and the environm ...
Ppt19(PS8)_Thermo_Hess
... What is the final temperature in a squeezed cold pack that contains 50.0 g of NH4NO3 dissolved in 125 mL of water? Assume the specific heat capacity of the dissolved NH4NO3 is negligible compared to water, an initial temperature of 25.0 C, and no heat transfer between the cold pack and the environm ...
... What is the final temperature in a squeezed cold pack that contains 50.0 g of NH4NO3 dissolved in 125 mL of water? Assume the specific heat capacity of the dissolved NH4NO3 is negligible compared to water, an initial temperature of 25.0 C, and no heat transfer between the cold pack and the environm ...
Scientific Principles: Chemical Properties
... • Consist of only one component with definite physical and chemical properties • Have the same composition throughout – E x a m p l e : O2 o r p u r e w a t e r ...
... • Consist of only one component with definite physical and chemical properties • Have the same composition throughout – E x a m p l e : O2 o r p u r e w a t e r ...
Document
... Standard enthalpy of formation (DH0f) is the heat change that results when one mole of a compound is formed from its elements at a pressure of 1 atm. The standard enthalpy of formation of any element in its most stable form is zero. 0 (C, graphite) = 0 ...
... Standard enthalpy of formation (DH0f) is the heat change that results when one mole of a compound is formed from its elements at a pressure of 1 atm. The standard enthalpy of formation of any element in its most stable form is zero. 0 (C, graphite) = 0 ...
Chapter 5 Review
... Enthalpy (H) - the heat flow into or out of a system in a process that occurs at constant pressure. DH = heat given off or absorbed during a reaction at constant pressure ...
... Enthalpy (H) - the heat flow into or out of a system in a process that occurs at constant pressure. DH = heat given off or absorbed during a reaction at constant pressure ...
Chemistry - Pearson School
... cannot learn merely by observing; you must be a participant. In particular, try to resist checking the Student Solutions Manual (if you have one) until you have made a sincere effort to solve the exercise yourself. If you get stuck on an exercise, however, get help from your teacher or another stude ...
... cannot learn merely by observing; you must be a participant. In particular, try to resist checking the Student Solutions Manual (if you have one) until you have made a sincere effort to solve the exercise yourself. If you get stuck on an exercise, however, get help from your teacher or another stude ...
Chapter 3
... – identity of reactants [R] and products [P]; use study of nomenclature to write equations – Identify the state of matter for each [R] and [P] – identify reaction type ...
... – identity of reactants [R] and products [P]; use study of nomenclature to write equations – Identify the state of matter for each [R] and [P] – identify reaction type ...
Balancing ANY chemical Equation
... The solubility guidelines may be used to predict whether a precipitation reaction will occur. When solutions of soluble ionic compounds are mixed together we need to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble compound ...
... The solubility guidelines may be used to predict whether a precipitation reaction will occur. When solutions of soluble ionic compounds are mixed together we need to identify all the ions present in the solution and then consider if possible cation/anion pairing could result in an insoluble compound ...
Document
... The entropy change accompanying any physical or chemical transformation approaches zero as the temperature approaches zero: ΔS 0 as T 0 provided all the substances involved are perfectly crystalline. ...
... The entropy change accompanying any physical or chemical transformation approaches zero as the temperature approaches zero: ΔS 0 as T 0 provided all the substances involved are perfectly crystalline. ...
Chemical Reactions PPT
... • Coefficient-represent the number of units of each substance taking part in the reaction ...
... • Coefficient-represent the number of units of each substance taking part in the reaction ...
IB Physics
... Second Law of Thermodynamics: It is impossible to extract an amount of heat QH from a hot reservoir and use it all to do work W . Some amount of heat QC must be exhausted to a cold reservoir. ...
... Second Law of Thermodynamics: It is impossible to extract an amount of heat QH from a hot reservoir and use it all to do work W . Some amount of heat QC must be exhausted to a cold reservoir. ...
Why Study Chemistry
... • The capacity of something to do work – chemical, mechanical, thermal, electrical, radiant, sound, nuclear • The SI unit of energy is the Joule (J) – Other common units are • Calories (cal) • Kilowatt-hour (kW.hr) ...
... • The capacity of something to do work – chemical, mechanical, thermal, electrical, radiant, sound, nuclear • The SI unit of energy is the Joule (J) – Other common units are • Calories (cal) • Kilowatt-hour (kW.hr) ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.