TOPIC 11 Further equilibrium 11.1 Chemical equilibrium
... Note: It is essential to show the division by the volume, V dm3, even though they cancel, to show that you have understood that the terms in the expression are concentrations. Also, it is essential to state that there are no units, since the question demands that you state the units, if any. Lastly, ...
... Note: It is essential to show the division by the volume, V dm3, even though they cancel, to show that you have understood that the terms in the expression are concentrations. Also, it is essential to state that there are no units, since the question demands that you state the units, if any. Lastly, ...
General Chemistry 1 and 2
... use the gas laws to determine pressure, volume, or temperature of a gas under certain conditions of change 4. use the ideal gas equation to calculate pressure, volume, temperature, or number of moles of a gas 5. use Dalton’s law of partial pressures to relate mole fraction and partial pressure of ga ...
... use the gas laws to determine pressure, volume, or temperature of a gas under certain conditions of change 4. use the ideal gas equation to calculate pressure, volume, temperature, or number of moles of a gas 5. use Dalton’s law of partial pressures to relate mole fraction and partial pressure of ga ...
DELTAHPP
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
enthalpy changes
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
No Slide Title
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
... selected topics at AS and A2 level Chemistry. It is based on the requirements of the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board ...
CHAPTER 10-11 PRACTICE FOR TEST Answer Section
... ____ 29. What is the volume, in liters, of 0.500 mol of C H gas at STP? a. 0.0335 L c. 16.8 L b. 11.2 L d. 22.4 L ____ 30. If the density of an unknown gas Z is 4.50 g/L at STP, what is the molar mass of gas Z? a. 0.201 g/mol c. 26.9 g/mol b. 5.00 g/mol d. 101 g/mol ____ 31. If 60.2 grams of Hg comb ...
... ____ 29. What is the volume, in liters, of 0.500 mol of C H gas at STP? a. 0.0335 L c. 16.8 L b. 11.2 L d. 22.4 L ____ 30. If the density of an unknown gas Z is 4.50 g/L at STP, what is the molar mass of gas Z? a. 0.201 g/mol c. 26.9 g/mol b. 5.00 g/mol d. 101 g/mol ____ 31. If 60.2 grams of Hg comb ...
ENZYMES
... – Lipase works only on __________ – Sucrase works only on ____________ – Protease works only on ____________ – __________ works only on fructose ...
... – Lipase works only on __________ – Sucrase works only on ____________ – Protease works only on ____________ – __________ works only on fructose ...
APPLICATION OF IONIC LIQUIDS IN ORGANIC SYNTHESIS
... methodologies. These activities may provide more information on the understanding of mechanisms of organic reactions using ionic liquids as reaction media, which have been traditionally conducted in molecular solvents. It may also provide improved methodologies for product separations, particularly ...
... methodologies. These activities may provide more information on the understanding of mechanisms of organic reactions using ionic liquids as reaction media, which have been traditionally conducted in molecular solvents. It may also provide improved methodologies for product separations, particularly ...
Chemistry
... aspects of general chemistry. Chemistry is mastered when students make the right connections in three key areas: topics that are related, conceptual reasoning with quantitative work, and the different modes of communicating information. McMurry/Fay’s Chemistry, Sixth Edition breaks through the tradi ...
... aspects of general chemistry. Chemistry is mastered when students make the right connections in three key areas: topics that are related, conceptual reasoning with quantitative work, and the different modes of communicating information. McMurry/Fay’s Chemistry, Sixth Edition breaks through the tradi ...
x - mrs. leinweber`s wiki
... hydrogen. Evidence indicates that this reaction establishes an equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed in the vessel. Kc for this reaction is 4.20 at 900C. Calculate the concentration of each substance at equilibrium. ...
... hydrogen. Evidence indicates that this reaction establishes an equilibrium with only partial conversion of reactants to products. Initially, 2.00 mol of each reactant is placed in the vessel. Kc for this reaction is 4.20 at 900C. Calculate the concentration of each substance at equilibrium. ...
Chemistry 21A: Survey of General and Organic Chemistry
... include questions on material covered only in Lecture (i.e. not discussed in the texts). Consistent late arrival may result in a lower grade; MORE THAN FOUR ABSENCES may result in being dropped from the class. If you arrive to LAB after discussion of safety procedures you may be excluded from the la ...
... include questions on material covered only in Lecture (i.e. not discussed in the texts). Consistent late arrival may result in a lower grade; MORE THAN FOUR ABSENCES may result in being dropped from the class. If you arrive to LAB after discussion of safety procedures you may be excluded from the la ...
Post Lab Questions
... Conduct yourself in a responsible manner at all times in the laboratory. Follow all written and verbal instructions carefully. If you do not understand a direction or part of the procedure, ask the instructor before proceeding. Never work alone. No student may work in the laboratory without an instr ...
... Conduct yourself in a responsible manner at all times in the laboratory. Follow all written and verbal instructions carefully. If you do not understand a direction or part of the procedure, ask the instructor before proceeding. Never work alone. No student may work in the laboratory without an instr ...
Thermodynamics of Ion Association in the Saturated Solution of
... an ion upon its transfer from water to an organic–aqueous mixture. Such quantities are usually “well behaved” in the sense that they vary smoothly as a function of solvent composition, even though they may show great diversity. It was concluded that the Gibbs free energies of transfer increase in ne ...
... an ion upon its transfer from water to an organic–aqueous mixture. Such quantities are usually “well behaved” in the sense that they vary smoothly as a function of solvent composition, even though they may show great diversity. It was concluded that the Gibbs free energies of transfer increase in ne ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
Stoichiometry: Calculations with Chemical Formulas and
... Molar Mass • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... Molar Mass • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
Massachusetts Tests for Educator Licensure (MTEL )
... flammability. This information is needed in the event of an accident, such as a spill, ingestion, etc. A is incorrect because the room number of the laboratory is not information that is needed in the event of an accident. B is incorrect because the date the stock reagent bottle was received is not ...
... flammability. This information is needed in the event of an accident, such as a spill, ingestion, etc. A is incorrect because the room number of the laboratory is not information that is needed in the event of an accident. B is incorrect because the date the stock reagent bottle was received is not ...
View Full Text
... system. Model predictions show that protonated piperazine is the dominant species at PCO2* > 250 Pa. This is significant due to the decreased amount of free amine available for reaction with CO2, potentially detracting from the rate of CO2 absorption. In contrast, the 3.6 m K+/0.6 m PZ solution cont ...
... system. Model predictions show that protonated piperazine is the dominant species at PCO2* > 250 Pa. This is significant due to the decreased amount of free amine available for reaction with CO2, potentially detracting from the rate of CO2 absorption. In contrast, the 3.6 m K+/0.6 m PZ solution cont ...
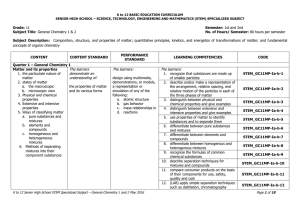
Table of Contents Pages Unit 1- Matter and Change 1
... measure of the amount of matter that an object contains. Virtually all of the matter around us consists of mixtures. A mixture can be defined as something that has _____________________ composition. Soda is a mixture (carbon dioxide is dissolved in it), and ____________________ is a mixture (it can ...
... measure of the amount of matter that an object contains. Virtually all of the matter around us consists of mixtures. A mixture can be defined as something that has _____________________ composition. Soda is a mixture (carbon dioxide is dissolved in it), and ____________________ is a mixture (it can ...
deltahpps
... Always only ONE MOLE of what you are burning on the LHS of the equation To aid balancing the equation, remember... you get one carbon dioxide molecule for every carbon atom in the original and one water molecule for every two hydrogen atoms When you have done this, go back and balance the oxygen. ...
... Always only ONE MOLE of what you are burning on the LHS of the equation To aid balancing the equation, remember... you get one carbon dioxide molecule for every carbon atom in the original and one water molecule for every two hydrogen atoms When you have done this, go back and balance the oxygen. ...
Equilibrium - pedagogics.ca
... hydrogen iodide change with time, we should obtain a graph of the form shown in Figure 7.3. The concentration of H2 decreases at first, until it levels off as equilibrium is reached. The concentration of HI is initially zero, but it increases until it flattens off and does not change any more as equ ...
... hydrogen iodide change with time, we should obtain a graph of the form shown in Figure 7.3. The concentration of H2 decreases at first, until it levels off as equilibrium is reached. The concentration of HI is initially zero, but it increases until it flattens off and does not change any more as equ ...
Buffer Capacity
... About the only time a buffer solution is used in its pure state, without any threat to its composition and, therefore pH value, is in standardizing a pH meter. The immersion of a glass electrode does not contaminate the buffer in the slightest, so the pH is completely unaffected. Most of the time bu ...
... About the only time a buffer solution is used in its pure state, without any threat to its composition and, therefore pH value, is in standardizing a pH meter. The immersion of a glass electrode does not contaminate the buffer in the slightest, so the pH is completely unaffected. Most of the time bu ...
Table of Contents
... A physical change is a change in matter that does not involve a change in the chemical identity of individual substances. The matter only changes in appearance. Examples: ______________, _________________, __________________, _________________, ___________________, and _____________________. A chemi ...
... A physical change is a change in matter that does not involve a change in the chemical identity of individual substances. The matter only changes in appearance. Examples: ______________, _________________, __________________, _________________, ___________________, and _____________________. A chemi ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.