Experimental and Computational Evidence of Metal‑O2 Activation
... prepared from the apoprotein following an optimized protocol.25 Chemicals were obtained from commercial sources in the highest grade available. Specifically deuterated βphenethylamine (PEA) and benzylamine (BA) were supplied by C/D/N isotopes. D2O was supplied by Cambridge Isotopes Laboratories and H ...
... prepared from the apoprotein following an optimized protocol.25 Chemicals were obtained from commercial sources in the highest grade available. Specifically deuterated βphenethylamine (PEA) and benzylamine (BA) were supplied by C/D/N isotopes. D2O was supplied by Cambridge Isotopes Laboratories and H ...
Chapter 15 Chemical Equilibrium
... • calculate Q and predict the direction of a reaction by relating Q to Keq • calculate equilibrium concentrations of reactants and/or products when given Keq Equilibrium ...
... • calculate Q and predict the direction of a reaction by relating Q to Keq • calculate equilibrium concentrations of reactants and/or products when given Keq Equilibrium ...
Review Packet Answers - Bremerton School District
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
Ciprofloxacin Hcl (Cas No 86393-32-0)
... Ciprofloxacin is 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C17H18FN3O3 and its molecular weight is 331.4. It is a faintly yellowish to light yellow crystalline substance and its chemical structure is as follows: ...
... Ciprofloxacin is 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C17H18FN3O3 and its molecular weight is 331.4. It is a faintly yellowish to light yellow crystalline substance and its chemical structure is as follows: ...
EQUILIBRIUM
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
EQUILIBRIUM
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
PDF w - ACS Publications - American Chemical Society
... photolysis of [Cp(CO)2Os]2 in C6D6 or THF-d8 in the presence of excess 1,4-cyclohexadiene led to the formation of the osmium hydride, Cp(CO)2OsH in 83−88% yield, together with benzene. Interestingly, the photolysis of [Cp(CO)2Os]2 in the absence of CHD in THF also afforded Cp(CO)2OsH in ∼30% yield. T ...
... photolysis of [Cp(CO)2Os]2 in C6D6 or THF-d8 in the presence of excess 1,4-cyclohexadiene led to the formation of the osmium hydride, Cp(CO)2OsH in 83−88% yield, together with benzene. Interestingly, the photolysis of [Cp(CO)2Os]2 in the absence of CHD in THF also afforded Cp(CO)2OsH in ∼30% yield. T ...
STOICHIOMETRY
... 1. Under appropriate reaction conditions copper and oxygen react to form copper II oxide. In an experiment with 45.3 g copper and an excess of oxygen, a percent yield of 96.7 % was obtained. What is the actual yield of copper II oxide in grams? Ans: 56.7 g 2. The percent yield for the reaction : PCl ...
... 1. Under appropriate reaction conditions copper and oxygen react to form copper II oxide. In an experiment with 45.3 g copper and an excess of oxygen, a percent yield of 96.7 % was obtained. What is the actual yield of copper II oxide in grams? Ans: 56.7 g 2. The percent yield for the reaction : PCl ...
Stoichiometry - coercingmolecules
... of sodium ascorbate are present? c. How many moles of C are present? d. How many moles of Na are present? e. How many formula units of sodium ascorbate are present? f. How many atoms of Na are present? ...
... of sodium ascorbate are present? c. How many moles of C are present? d. How many moles of Na are present? e. How many formula units of sodium ascorbate are present? f. How many atoms of Na are present? ...
Chapter 4 - KFUPM Faculty List
... One mole of H2O(g) at 1.00 atm and 100. oC occupies a volume of 30.6 L. If one mole of H2O(g) is condensed to one mole of H2O(l) at the same temperature and pressure, what would be ΔE for the condensation? (Density of water at 1.00 atm and 100. ...
... One mole of H2O(g) at 1.00 atm and 100. oC occupies a volume of 30.6 L. If one mole of H2O(g) is condensed to one mole of H2O(l) at the same temperature and pressure, what would be ΔE for the condensation? (Density of water at 1.00 atm and 100. ...
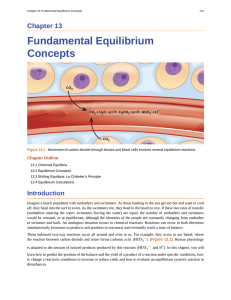
Fundamental Equilibrium Concepts
... exhausted and will reverse only under certain conditions. Such reactions are often depicted with a one-way arrow from reactants to products. Many other reactions, such as the formation of NO2 from N2O4, are reversible under more easily obtainable conditions and, therefore, are named as such. In a re ...
... exhausted and will reverse only under certain conditions. Such reactions are often depicted with a one-way arrow from reactants to products. Many other reactions, such as the formation of NO2 from N2O4, are reversible under more easily obtainable conditions and, therefore, are named as such. In a re ...
Quantitative Comparison of the Hydrogen Bond
... Comparison of the H-Bond Network in the A-State and in the NatiVe State. The transition from the native state to the A-state leads to a very striking reshuffling of the H-bond network in ubiquitin, which can be followed in detail by the h3 JNC′ scalar correlations between H-bond donor and acceptor a ...
... Comparison of the H-Bond Network in the A-State and in the NatiVe State. The transition from the native state to the A-state leads to a very striking reshuffling of the H-bond network in ubiquitin, which can be followed in detail by the h3 JNC′ scalar correlations between H-bond donor and acceptor a ...
BSC with Chemistry CBCS Syllabus 2016-17
... (MCQ/true and false / fill in the blanks etc.) of one mark each covering the entire paper. ...
... (MCQ/true and false / fill in the blanks etc.) of one mark each covering the entire paper. ...
An Introduction to Chemical Science
... either to do the work or to recite the lesson. In the laboratory each pupil has a locker under his table, furnished with apparatus, as specified in the Appendix. Each has also the author's "Laboratory Manual," which contains on every left-hand page full directions for an experiment, with observation ...
... either to do the work or to recite the lesson. In the laboratory each pupil has a locker under his table, furnished with apparatus, as specified in the Appendix. Each has also the author's "Laboratory Manual," which contains on every left-hand page full directions for an experiment, with observation ...
chem textbook 2015 - Manitowoc Public School District
... The intent of the following information is to give answers and suggestions to questions that students often ask, it is meant to work in conjunction with Suggestions for Boosting Grades. “It makes sense in class but not when I get home.” This generally means that your notes are incomplete, meaning th ...
... The intent of the following information is to give answers and suggestions to questions that students often ask, it is meant to work in conjunction with Suggestions for Boosting Grades. “It makes sense in class but not when I get home.” This generally means that your notes are incomplete, meaning th ...
Chapter 7 - NordoniaHonorsChemistry
... Example 7.7—When an Aqueous Solution of Sodium Carbonate Is Added to an Aqueous Solution of Copper(II) Chloride, a White Solid Forms. Write the formulas of the reactants and Determine the ions present when each reactant dissociates. ...
... Example 7.7—When an Aqueous Solution of Sodium Carbonate Is Added to an Aqueous Solution of Copper(II) Chloride, a White Solid Forms. Write the formulas of the reactants and Determine the ions present when each reactant dissociates. ...
Chapter 1 – Reaction Kinetics Answer Key
... change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. The amount (number of moles) certainly does change. However, we must realize that ...
... change (appreciably for the liquid if it is the solvent and at all for the solid) during a chemical reaction. The amount (number of moles) certainly does change. However, we must realize that ...
Mole
... Mole Ratio In a balanced equation, the ration between the numbers of moles of any two substances. ...
... Mole Ratio In a balanced equation, the ration between the numbers of moles of any two substances. ...
Chemistry
... 54. Which of the following reagents can convert acetic acid into ethanol ? (A) Sn + HCl (B) H2 + Pt (C) LiAlH4 + ether (D) H2 + Ni 55. An organic compound ‘X’ is oxidized by using acidified K2Cr2O7. The product obtained reacts with phenyl hydrazine but does not give silver mirror test. The possible ...
... 54. Which of the following reagents can convert acetic acid into ethanol ? (A) Sn + HCl (B) H2 + Pt (C) LiAlH4 + ether (D) H2 + Ni 55. An organic compound ‘X’ is oxidized by using acidified K2Cr2O7. The product obtained reacts with phenyl hydrazine but does not give silver mirror test. The possible ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.