Chemistry, Biology
... dominated physics for about 2000 years. The greatest contribution to the development of mechanics is by one of the greatest physicists of all time, Isaac Newton. By extending Galileo’s methods and understanding of motion and gravitation, Newton developed the three laws of motion and his law of unive ...
... dominated physics for about 2000 years. The greatest contribution to the development of mechanics is by one of the greatest physicists of all time, Isaac Newton. By extending Galileo’s methods and understanding of motion and gravitation, Newton developed the three laws of motion and his law of unive ...
2 CHEMICAL ARITHMATICS W MODULE - 1
... oxide. (The volume relationship becomes more useful for reactions involving 2 or more gaseous substances). *Earlier, the standard pressure was taken as 1 atmosphere and the volume of one mole of gas at STP was taken as 22.4 L. ...
... oxide. (The volume relationship becomes more useful for reactions involving 2 or more gaseous substances). *Earlier, the standard pressure was taken as 1 atmosphere and the volume of one mole of gas at STP was taken as 22.4 L. ...
the principles of thermodynamics by n.d. hari dass
... recently, biology and even black holes. The Principles of Thermodynamics offers a fresh perspective on classical thermodynamics, highlighting its elegance, power, and conceptual economy. The book demonstrates how much of natural phenomena can be understood through thermodynamics. ...
... recently, biology and even black holes. The Principles of Thermodynamics offers a fresh perspective on classical thermodynamics, highlighting its elegance, power, and conceptual economy. The book demonstrates how much of natural phenomena can be understood through thermodynamics. ...
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical
... while the “surroundings” is EVERYTHING else. Suppose we burn gasoline in an internal combustion engine. The gasoline (and air) in the cylinder(s) composes the “system”, while the engine, and the air contacting the engine is the “surroundings”. Together the “system” and “surroundings” compose the “un ...
... while the “surroundings” is EVERYTHING else. Suppose we burn gasoline in an internal combustion engine. The gasoline (and air) in the cylinder(s) composes the “system”, while the engine, and the air contacting the engine is the “surroundings”. Together the “system” and “surroundings” compose the “un ...
Corium z157
... high heat or any inherent weakness of ordinary lubricants. • Corium Z157 superior quality "dry" formulation is based on "indestructible" molybdenum disulphide (MD) particles. • Corium Z157 withstands extreme temperatures – up to 1112˚F (600˚C) – and severe chemical corrosion. • Corium Z157 eliminate ...
... high heat or any inherent weakness of ordinary lubricants. • Corium Z157 superior quality "dry" formulation is based on "indestructible" molybdenum disulphide (MD) particles. • Corium Z157 withstands extreme temperatures – up to 1112˚F (600˚C) – and severe chemical corrosion. • Corium Z157 eliminate ...
Nickel catalyst auto-reduction during steam reforming of bio
... and H2 and reduced Ni but do not represent the actual, more complex mechanism involving adsorption of reactants, dissociation and formation of intermediates on the catalyst surface, recombination reactions, and desorption of products from the catalyst. In particular, reaction R2 is chosen here with ...
... and H2 and reduced Ni but do not represent the actual, more complex mechanism involving adsorption of reactants, dissociation and formation of intermediates on the catalyst surface, recombination reactions, and desorption of products from the catalyst. In particular, reaction R2 is chosen here with ...
Chemistry booklet
... What about the following PHOSPHO-GLYCERIDE molecule containing both hydrophilic ( water-loving) and hydro-phobic ( water-hating ) regions ( termed an amphi-philic molecule) ? ...
... What about the following PHOSPHO-GLYCERIDE molecule containing both hydrophilic ( water-loving) and hydro-phobic ( water-hating ) regions ( termed an amphi-philic molecule) ? ...
analytical chemistry - Львівський національний медичний
... Requirements (demands) to analytical reactions: 1) reaction must run quickly, in practice – immediately; 2) reaction must accompanied with accordance (special) analytical effect; 3) reaction must be irreversible – run in one way (in one side); 4) reaction must have high specificity and have high sen ...
... Requirements (demands) to analytical reactions: 1) reaction must run quickly, in practice – immediately; 2) reaction must accompanied with accordance (special) analytical effect; 3) reaction must be irreversible – run in one way (in one side); 4) reaction must have high specificity and have high sen ...
Stoichiometry: Calculations with Chemical Formulas and
... Molar Mass • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
... Molar Mass • By definition, these are the mass of 1 mol of a substance (i.e., g/mol) – The molar mass of an element is the mass number for the element that we find on the periodic table – The formula weight (in amu’s) will be the same number as the molar mass (in g/mol) Stoichiometry ...
EOCT Physical Science Study Guide August 2008
... chair, and take a few deep breaths. Then, sit up straight, pick up your pencil, and begin to concentrate on the test again. Remember that each test section is only 60 minutes. Use positive self-talk. If you find yourself saying negative things to yourself, such as “I can’t pass this test,” it is imp ...
... chair, and take a few deep breaths. Then, sit up straight, pick up your pencil, and begin to concentrate on the test again. Remember that each test section is only 60 minutes. Use positive self-talk. If you find yourself saying negative things to yourself, such as “I can’t pass this test,” it is imp ...
GCE Chemistry Specification (From 2015 - WALES ONLY
... find arithmetic means by calculating relative atomic mass from mass spectrum data use ratios and percentages by solving empirical formula problems and calculating atom economy and yield of a reaction; recognise and make use of units in calculations involving amounts of substance; use powers in calcu ...
... find arithmetic means by calculating relative atomic mass from mass spectrum data use ratios and percentages by solving empirical formula problems and calculating atom economy and yield of a reaction; recognise and make use of units in calculations involving amounts of substance; use powers in calcu ...
Proposed syllabus and Scheme of Examination B.Sc. (Program) with Chemistry Submitted To
... Phases, components and degrees of freedom of a system, criteria of phase equilibrium. Gibbs Phase Rule and its thermodynamic derivation. Derivation of Clausius – Clapeyron equation and its importance in phase equilibria. Phase diagrams of one-componentsystems (water and sulphur) and two component s ...
... Phases, components and degrees of freedom of a system, criteria of phase equilibrium. Gibbs Phase Rule and its thermodynamic derivation. Derivation of Clausius – Clapeyron equation and its importance in phase equilibria. Phase diagrams of one-componentsystems (water and sulphur) and two component s ...
Stoichiometry
... formed. The reaction will stop when all of the limiting reactant is consumed. Example: I want to assemble a gadget that requires one nut, one bolt and two washers for every hole. I have in my garage a bucket filled with 12 washers, 4 bolts and five nuts. What is the LIMITING SMALL METAL ...
... formed. The reaction will stop when all of the limiting reactant is consumed. Example: I want to assemble a gadget that requires one nut, one bolt and two washers for every hole. I have in my garage a bucket filled with 12 washers, 4 bolts and five nuts. What is the LIMITING SMALL METAL ...
www.xtremepapers.net
... manipulation of apparatus, presentation of data, analysis and evaluation. Paper 5 is a written examination that will test the higher-order experimental skills of planning, analysis and evaluation. It should be stressed that candidates cannot be adequately prepared for this paper without extensive la ...
... manipulation of apparatus, presentation of data, analysis and evaluation. Paper 5 is a written examination that will test the higher-order experimental skills of planning, analysis and evaluation. It should be stressed that candidates cannot be adequately prepared for this paper without extensive la ...
Module 2
... these particles. The disorder in a system depends only on the conditions that determine the state of the system, such as composition, temperature, and pressure. The change in entropy therefore depends only on the initial and final states of the system. Entropy, like enthalpy is a state function. Def ...
... these particles. The disorder in a system depends only on the conditions that determine the state of the system, such as composition, temperature, and pressure. The change in entropy therefore depends only on the initial and final states of the system. Entropy, like enthalpy is a state function. Def ...
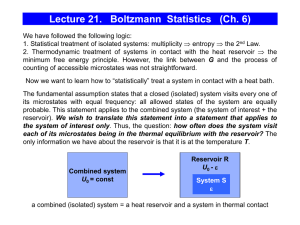
Lecture 21. Boltzmann Statistics (Ch. 6)
... U0 = UR+ US = const According to the fundamental assumption of thermodynamics, all the states of the combined (isolated) system “R+S” are equally probable. By specifying the microstate of the system k, we have reduced S to 1 and SS to 0. Thus, the probability of occurrence of a situation where the ...
... U0 = UR+ US = const According to the fundamental assumption of thermodynamics, all the states of the combined (isolated) system “R+S” are equally probable. By specifying the microstate of the system k, we have reduced S to 1 and SS to 0. Thus, the probability of occurrence of a situation where the ...
Chapter 4 Chemical Reactions and Solution Stoichiometry 4.1
... Notice that classification of ionic compound solubility is primarily based on the anion in the compound. When classifying an ionic compound as soluble or insoluble, first determine whether the anion present is typically found in soluble or insoluble compounds. Next, see if the cation in the compound ...
... Notice that classification of ionic compound solubility is primarily based on the anion in the compound. When classifying an ionic compound as soluble or insoluble, first determine whether the anion present is typically found in soluble or insoluble compounds. Next, see if the cation in the compound ...
Gas-Phase Reactions of Fe (CH2O)+ and Fe (CH2S)+ with Small
... been the focus of intense investigation for the past 20 years, yielding a great deal of information on “intrinsic” properties, such as kinetics, thermochemistry, and reaction mechanisms in the absence of solvation and counterion effects.1 The reactions with simple hydrocarbons have been particularly ...
... been the focus of intense investigation for the past 20 years, yielding a great deal of information on “intrinsic” properties, such as kinetics, thermochemistry, and reaction mechanisms in the absence of solvation and counterion effects.1 The reactions with simple hydrocarbons have been particularly ...
ExamView - 1999 AP Chemistry Exam.tst
... Directions: Each set of lettered choices below refers to the numbered questions or statements immediately following it. Select the one lettered choice that best answers each question or best fits each statement and then fill in the corresponding oval on the answer sheet. A choice may be used once, m ...
... Directions: Each set of lettered choices below refers to the numbered questions or statements immediately following it. Select the one lettered choice that best answers each question or best fits each statement and then fill in the corresponding oval on the answer sheet. A choice may be used once, m ...
Sustainable Oxidation Catalysis for Synthesis
... * corresponding author: [email protected] Abstract At present, many oxidation reactions rely on inefficient and wasteful methods that are problematic on a larger scale. There is a need to develop efficient catalysts that use sustainable terminal oxidants such as molecular oxygen or hydrogen pero ...
... * corresponding author: [email protected] Abstract At present, many oxidation reactions rely on inefficient and wasteful methods that are problematic on a larger scale. There is a need to develop efficient catalysts that use sustainable terminal oxidants such as molecular oxygen or hydrogen pero ...
Supporting Information for Angew. Chem. Int. Ed. Z52444 © Wiley
... Unless stated otherwise, reactions were performed in oven-dried glassware, under an atmosphere of oxygen, using freshly distilled solvents. Although we have never experienced an accident, all reactions must be performed with appropriate caution in a fume hood due to the flammable nature of mixtures ...
... Unless stated otherwise, reactions were performed in oven-dried glassware, under an atmosphere of oxygen, using freshly distilled solvents. Although we have never experienced an accident, all reactions must be performed with appropriate caution in a fume hood due to the flammable nature of mixtures ...
Chemical thermodynamics

Chemical thermodynamics is the study of the interrelation of heat and work with chemical reactions or with physical changes of state within the confines of the laws of thermodynamics. Chemical thermodynamics involves not only laboratory measurements of various thermodynamic properties, but also the application of mathematical methods to the study of chemical questions and the spontaneity of processes.The structure of chemical thermodynamics is based on the first two laws of thermodynamics. Starting from the first and second laws of thermodynamics, four equations called the ""fundamental equations of Gibbs"" can be derived. From these four, a multitude of equations, relating the thermodynamic properties of the thermodynamic system can be derived using relatively simple mathematics. This outlines the mathematical framework of chemical thermodynamics.