Researchers have now found an explanation for
... ‘The low ratio of 18O to 16O contents in the crystal rims indicate that something in the magmatic system changed drastically just before the big eruption. The explanation behind these chemical signatures is that the magma melted and assimilated a large volume of a local rock that itself is character ...
... ‘The low ratio of 18O to 16O contents in the crystal rims indicate that something in the magmatic system changed drastically just before the big eruption. The explanation behind these chemical signatures is that the magma melted and assimilated a large volume of a local rock that itself is character ...
Tellurium crystals - Iowa Research Online
... hydrogen, and is actual size. It is highly important that the temperature of formation of the crystals should be as nearly constant as possible, because crystals formed at different temperatures, and consequently different pressures, show varying results, and if, therefore, a single crystal be the s ...
... hydrogen, and is actual size. It is highly important that the temperature of formation of the crystals should be as nearly constant as possible, because crystals formed at different temperatures, and consequently different pressures, show varying results, and if, therefore, a single crystal be the s ...
Solutions
... Solutions have one or more solutes (dissolvee) and a solvent (dissolver). When a substance dissolves it is soluble Liquids that mix are miscible, those that don't are immiscible ...
... Solutions have one or more solutes (dissolvee) and a solvent (dissolver). When a substance dissolves it is soluble Liquids that mix are miscible, those that don't are immiscible ...
Experimental set-up for particle detection in solid crystals of inert
... Crystals made of inert gasses solidified at cryogenic temperature have been used since the 50’s to study reactive species in a low interacting environment 1,2 . In this technique, named matrix isolation spectroscopy, the guest particles (atoms, molecules or ions) are embedded in a continuous matrix ...
... Crystals made of inert gasses solidified at cryogenic temperature have been used since the 50’s to study reactive species in a low interacting environment 1,2 . In this technique, named matrix isolation spectroscopy, the guest particles (atoms, molecules or ions) are embedded in a continuous matrix ...
Texture of Igneous Rocks
... this texture is a result of two stages of cooling; 1) slow cooling forming the larger crystals. 2) rapid cooling forming the finer crystals. ...
... this texture is a result of two stages of cooling; 1) slow cooling forming the larger crystals. 2) rapid cooling forming the finer crystals. ...
practice test2
... Which of the following molecules can form hydrogen bonds A) CH4 B) NaH C) NH3 D) BH3 ...
... Which of the following molecules can form hydrogen bonds A) CH4 B) NaH C) NH3 D) BH3 ...
DATOLITE - Celestial Earth Minerals
... HISTORY, NAME, LOCALITIES: Datolite, pronounced DATE-oh-lite, was recognized as a mineral species and named in 1806. Its name derives from the Greek dateisthai, meaning “to divide” and alluding to the tendency of granular aggregates to crumble easily into bits. Notable collecting localities are in R ...
... HISTORY, NAME, LOCALITIES: Datolite, pronounced DATE-oh-lite, was recognized as a mineral species and named in 1806. Its name derives from the Greek dateisthai, meaning “to divide” and alluding to the tendency of granular aggregates to crumble easily into bits. Notable collecting localities are in R ...
GEOS 306 Mineralogy — Lecture Summary for Final Exam
... Short answer, matching, fill in the blank May include identifying or labeling diagrams 200 points total + 20 points extra credit ...
... Short answer, matching, fill in the blank May include identifying or labeling diagrams 200 points total + 20 points extra credit ...
Elements Compounds Mixtures
... • Two or more substances evenly mixed. • You can NOT see the different substances, even with a microscope! • Examples: gatorade, salt water, brass, air ...
... • Two or more substances evenly mixed. • You can NOT see the different substances, even with a microscope! • Examples: gatorade, salt water, brass, air ...
The periodical trends of elements in chemical properties
... Additional Chapter: The periodical trends of elements in chemical properties Mendeleev’s periodic table In 1869 Dmitri Mendeleev (1834-1907) succeeded in organizing the 62 elements known at that time into a system of rows and columns on the basis of increasing mass and similar chemical and physical ...
... Additional Chapter: The periodical trends of elements in chemical properties Mendeleev’s periodic table In 1869 Dmitri Mendeleev (1834-1907) succeeded in organizing the 62 elements known at that time into a system of rows and columns on the basis of increasing mass and similar chemical and physical ...
Physical properties of Semiconductors
... METHODS OF INSTRUCTION REQUIREMENTS AND ASSESSMENTS GRADING SYSTEM TOTAL STUDENT WORKLOAD NEEDED TO ACHIEVE EXPECTED LEARNING OUTCOMES EXPRESSED IN TIME AND ECTS CREDIT POINTS ...
... METHODS OF INSTRUCTION REQUIREMENTS AND ASSESSMENTS GRADING SYSTEM TOTAL STUDENT WORKLOAD NEEDED TO ACHIEVE EXPECTED LEARNING OUTCOMES EXPRESSED IN TIME AND ECTS CREDIT POINTS ...
What is a mineral? - The Science Queen
... orderly pattern (Crystals) Crystalline means that atoms are arranged in a pattern that is repeated over and over again. For example, graphite’s atoms are arranged in layers and halite's are arranged in cubes. The crystal system a mineral has can help a geologist identify the mineral. ...
... orderly pattern (Crystals) Crystalline means that atoms are arranged in a pattern that is repeated over and over again. For example, graphite’s atoms are arranged in layers and halite's are arranged in cubes. The crystal system a mineral has can help a geologist identify the mineral. ...
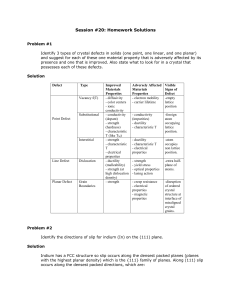
Session #20: Homework Solutions
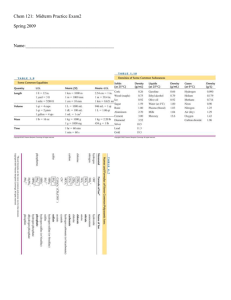
... A cubic metal (r = 0.77 Å) exhibits plastic deformation by slip along <111> directions. Determine its planar packing density (atoms/m2) for its densest family of planes. Solution Slip along <111> directions suggests a BCC system, corresponding to {110}, <111> slip. Therefore: a 3 = 4r ...
... A cubic metal (r = 0.77 Å) exhibits plastic deformation by slip along <111> directions. Determine its planar packing density (atoms/m2) for its densest family of planes. Solution Slip along <111> directions suggests a BCC system, corresponding to {110}, <111> slip. Therefore: a 3 = 4r ...
Solutions Foldable
... o Li2SO4, MgO, Na2O, Ca(OH)2 Now that we know what will mix to make a solution how do we know how MUCH solute can be used? Solubility Charts/Graphs! Solubility: the maximum amount of solute that will dissolve in a given amount of solvent at a given temperature. Based on solubility there are 3 kinds ...
... o Li2SO4, MgO, Na2O, Ca(OH)2 Now that we know what will mix to make a solution how do we know how MUCH solute can be used? Solubility Charts/Graphs! Solubility: the maximum amount of solute that will dissolve in a given amount of solvent at a given temperature. Based on solubility there are 3 kinds ...
Document
... they are deformed about 0.1% of their original dimensions. These crystals readily release the current when the orientation of crystal is disturbed by mechanical vibrations. These signals are in turn given to IC 741 (Op-Amp) ...
... they are deformed about 0.1% of their original dimensions. These crystals readily release the current when the orientation of crystal is disturbed by mechanical vibrations. These signals are in turn given to IC 741 (Op-Amp) ...
3-7-11 Aim: What is a Mineral?
... 2. What is a tetrahedron made up of? 3. What is the tetrahedron responsible for? ...
... 2. What is a tetrahedron made up of? 3. What is the tetrahedron responsible for? ...
الشريحة 1
... assemble into ordered arrays in one , two and three dimensions under the right conditions . b- Lattices of nano crystals consist of interacting • nano crystals and may exhibit novel Properties arising out of such interaction . c- Thus , the ability to engineer such assemblies • extends the reach of ...
... assemble into ordered arrays in one , two and three dimensions under the right conditions . b- Lattices of nano crystals consist of interacting • nano crystals and may exhibit novel Properties arising out of such interaction . c- Thus , the ability to engineer such assemblies • extends the reach of ...
Chemistry Final Study Guide
... 43. The three major categories of elements on the periodic table are the __________, __________, and __________. 44. The first group on the periodic table is called the __________ __________, and they are very reactive due to the fact that they tend to lose one __________. 45. Electrons in the outer ...
... 43. The three major categories of elements on the periodic table are the __________, __________, and __________. 44. The first group on the periodic table is called the __________ __________, and they are very reactive due to the fact that they tend to lose one __________. 45. Electrons in the outer ...
Optical Refrigeration: Researchers Achieve Milestone in
... allows us to minimize parasitic heat load from the environment. “We infer crystal temperature using a technique we developed that allows to measure temperature without making a contact with the sample, further avoiding unnecessary parasitic heat load on the sample. Combination of all of these ideas ...
... allows us to minimize parasitic heat load from the environment. “We infer crystal temperature using a technique we developed that allows to measure temperature without making a contact with the sample, further avoiding unnecessary parasitic heat load on the sample. Combination of all of these ideas ...
Mineralogy and Crystals
... negative charge due to loss or gain of electrons An Ionic Bond is formed by the attraction of oppositely charged ions – eg between Na+ and Cl- in NaCl (Halite) A Covalent Bond is formed where two atoms share an electron – eg between Si4+ and O2- in the silicate unit SiO4 Most minerals share both Ion ...
... negative charge due to loss or gain of electrons An Ionic Bond is formed by the attraction of oppositely charged ions – eg between Na+ and Cl- in NaCl (Halite) A Covalent Bond is formed where two atoms share an electron – eg between Si4+ and O2- in the silicate unit SiO4 Most minerals share both Ion ...
Paired with Lecture
... • We just studied Phase Diagrams which are thermodynamic maps which tell us the equilibrium phases present at any specific combination of temperature, pressure, and composition • These phase diagrams are based on the concept of Gibbs Free Energy, DG, which we have briefly introduced before: DG is ...
... • We just studied Phase Diagrams which are thermodynamic maps which tell us the equilibrium phases present at any specific combination of temperature, pressure, and composition • These phase diagrams are based on the concept of Gibbs Free Energy, DG, which we have briefly introduced before: DG is ...
Color of a mineral in its powdered form
... How are minerals identified? Physical properties Crystal Form • External expression of the crystal structure • Crystal growth is often interrupted because of competition for space and rapid loss of heat ...
... How are minerals identified? Physical properties Crystal Form • External expression of the crystal structure • Crystal growth is often interrupted because of competition for space and rapid loss of heat ...
Crystallization

Crystallization is the (natural or artificial) process of formation of solid crystals precipitating from a solution, melt or more rarely deposited directly from a gas. Crystallization is also a chemical solid–liquid separation technique, in which mass transfer of a solute from the liquid solution to a pure solid crystalline phase occurs. In chemical engineering crystallization occurs in a crystallizer. Crystallization is therefore an aspect of precipitation, obtained through a variation of the solubility conditions of the solute in the solvent, as compared to precipitation due to chemical reaction.