Study Island Copyright © 2012 Study Island
... B. The elements all share identical properties, but their properties are different than the compound's properties. C. The properties of a compound are different than the properties of its elements. D. The compound shares identical properties with one element, but all the other elements have differen ...
... B. The elements all share identical properties, but their properties are different than the compound's properties. C. The properties of a compound are different than the properties of its elements. D. The compound shares identical properties with one element, but all the other elements have differen ...
end of year review
... a. can be in any ratio. c. Retain their original identifying properties. b. can be separated easily. d. Chemically unite to form one substance. _____ 3. The graph below compares three states of a substance. ...
... a. can be in any ratio. c. Retain their original identifying properties. b. can be separated easily. d. Chemically unite to form one substance. _____ 3. The graph below compares three states of a substance. ...
template
... 4. When sodium hydroxide reacts with sulfuric acid (H2SO4), water and sodium sulfate are the products. Calculate the mass of sodium sulfate produced when 15.5 g of sodium hydroxide are reacted with 46.7 g of sulfuric acid. [Hint: which unit is used in all stoichiometry reasoning?] ...
... 4. When sodium hydroxide reacts with sulfuric acid (H2SO4), water and sodium sulfate are the products. Calculate the mass of sodium sulfate produced when 15.5 g of sodium hydroxide are reacted with 46.7 g of sulfuric acid. [Hint: which unit is used in all stoichiometry reasoning?] ...
Lesson 1 - Working With Chemicals
... For example, hydrogen has one electron and thus it wants to fill that orbit in order to become stable - so it will pair up with another hydrogen atom and they will share the two electrons – covalent bonding. ...
... For example, hydrogen has one electron and thus it wants to fill that orbit in order to become stable - so it will pair up with another hydrogen atom and they will share the two electrons – covalent bonding. ...
Honors Unit 3 - Stoichiometry
... 1. Zinc metal and aqueous lead (II) nitrate react to form aqueous zinc nitrate and solid lead. ...
... 1. Zinc metal and aqueous lead (II) nitrate react to form aqueous zinc nitrate and solid lead. ...
CAPE CHEMISTRY UNIT TWO REVISION PAPER MODULE 1 (a
... (TOTAL 15) Production of carbon dioxide is favoured by low temperature (since its production is exothermic), high pressure (since less gaseous molecules are present after its formation) and a faster air flow so that more oxygen is available for complete combustion. 2. (a) (i) State three different s ...
... (TOTAL 15) Production of carbon dioxide is favoured by low temperature (since its production is exothermic), high pressure (since less gaseous molecules are present after its formation) and a faster air flow so that more oxygen is available for complete combustion. 2. (a) (i) State three different s ...
NCEA Level 1 Chemistry (90933) 2014
... vigorously to form a salt and hydrogen gas. The magnesium reacts and disappears into solution; the solution warms up and there is fizzing due to production of hydrogen gas. Magnesium is high up on the activity series (above H), so will easily react. Copper is low on the activity series and there is ...
... vigorously to form a salt and hydrogen gas. The magnesium reacts and disappears into solution; the solution warms up and there is fizzing due to production of hydrogen gas. Magnesium is high up on the activity series (above H), so will easily react. Copper is low on the activity series and there is ...
42.89 KB
... 9. How much energy is required to transform 14.0 g ice at 0.0oC to steam at 110.0 oC? Given the following information: Specific heat capacities: ice, 2.1 J/g.oC; liquid, 4.2 J/g.oC; steam, 2.0 J/g.oC; ...
... 9. How much energy is required to transform 14.0 g ice at 0.0oC to steam at 110.0 oC? Given the following information: Specific heat capacities: ice, 2.1 J/g.oC; liquid, 4.2 J/g.oC; steam, 2.0 J/g.oC; ...
chapter 4 lecture slides
... The net ionic equation tells us: 1. changes in ionic strength (more ions present before the rxn than after) 2. what actually changed during a reaction Example: Cd2+ (aq) + S2-(aq) –> CdS (s) Writing ionic equations, ask: 1. is substance soluble ? 2. is substance a strong electrolyte? **If yes to bo ...
... The net ionic equation tells us: 1. changes in ionic strength (more ions present before the rxn than after) 2. what actually changed during a reaction Example: Cd2+ (aq) + S2-(aq) –> CdS (s) Writing ionic equations, ask: 1. is substance soluble ? 2. is substance a strong electrolyte? **If yes to bo ...
Tutorial 1
... 8. Identify the following as elements or compounds: NH3, N2, S8, NO, CO, CO2, H2, SO2 9. Give three number of protons and electrons in each of the following common ions: Na +, Ca2+, Al3+, Fe2+, I-, F-, S2-, O2-, N3-, K+, Mg2+, Fe3+, Br-, Mn2+, C4-, and Cu2+ 10. Define molecular formula and empirical ...
... 8. Identify the following as elements or compounds: NH3, N2, S8, NO, CO, CO2, H2, SO2 9. Give three number of protons and electrons in each of the following common ions: Na +, Ca2+, Al3+, Fe2+, I-, F-, S2-, O2-, N3-, K+, Mg2+, Fe3+, Br-, Mn2+, C4-, and Cu2+ 10. Define molecular formula and empirical ...
CHEM104 Examlette 1 – ANSWERS TOTAL POINTS = 94 Multiple
... How will this high pressure affect the phase transition from water to ice? (5 pts) Since the line marking the phase change between Solid and liquid phases has negative slope, it means that as P increases, the equilibrium of ice/water shifts to lower ( more negative) temperature. The m.p. decreases. ...
... How will this high pressure affect the phase transition from water to ice? (5 pts) Since the line marking the phase change between Solid and liquid phases has negative slope, it means that as P increases, the equilibrium of ice/water shifts to lower ( more negative) temperature. The m.p. decreases. ...
ouble Replacement or (Metathesis) Reactions
... forms at the negative electrode (cathode) and immediately undergoes reaction with water: ...
... forms at the negative electrode (cathode) and immediately undergoes reaction with water: ...
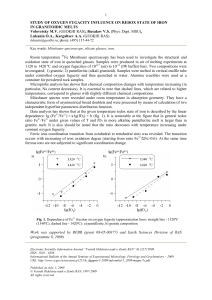
Study of oxygen fugacity influence on redox state of iron in
... Data analysis has shown that at the given temperature redox state of iron is described by the linear dependence: lg (Fe3+/Fe2+) = a·lg(fO2) + b (fig. 1). It is noticeable at the figure that in general redox ratio Fe3+/Fe2+ under given values of T and fO2 in more alkaline pantelleritic melt is larger ...
... Data analysis has shown that at the given temperature redox state of iron is described by the linear dependence: lg (Fe3+/Fe2+) = a·lg(fO2) + b (fig. 1). It is noticeable at the figure that in general redox ratio Fe3+/Fe2+ under given values of T and fO2 in more alkaline pantelleritic melt is larger ...
the ap chemistry summer assignment
... to do particular types of problems, you must really understand the chemistry and be able to apply it to different kinds of problems. AP Chemistry is a difficult course. To succeed you must keep up with the assignments and be willing to spend time working through the material. The College Board recom ...
... to do particular types of problems, you must really understand the chemistry and be able to apply it to different kinds of problems. AP Chemistry is a difficult course. To succeed you must keep up with the assignments and be willing to spend time working through the material. The College Board recom ...
Chemical Equations & Reactions
... Definition: a process by which 1 or more substances, called reactants, are changed into 1 or more substances, called products, with different physical & chemical properties. Evidence of a Chemical Reaction ...
... Definition: a process by which 1 or more substances, called reactants, are changed into 1 or more substances, called products, with different physical & chemical properties. Evidence of a Chemical Reaction ...
- Palisades School District
... The conjugate base of a weak acid reacts with water (hydrolysis) to reform the acid. Likewise, the conjugate acid of a weak base reacts with water to reform the base. ...
... The conjugate base of a weak acid reacts with water (hydrolysis) to reform the acid. Likewise, the conjugate acid of a weak base reacts with water to reform the base. ...
Chemistry Final Test 1999-2000 - Nashoba Valley Technical High
... Describe solvation – the dissolving process Compare solubility, concentration & saturation Describe factors that affect dissolving rate for solids & gases Describe & identify an electrolyte Interpret a solubility graph Chapter 15 – Acids and Bases Describe each of the following: 2 defini ...
... Describe solvation – the dissolving process Compare solubility, concentration & saturation Describe factors that affect dissolving rate for solids & gases Describe & identify an electrolyte Interpret a solubility graph Chapter 15 – Acids and Bases Describe each of the following: 2 defini ...
Exam only.
... the enthalpy of reaction is the difference between product and reactant enthalpies. the Gibbs free energy is a function of both enthalpy and entropy. ...
... the enthalpy of reaction is the difference between product and reactant enthalpies. the Gibbs free energy is a function of both enthalpy and entropy. ...
Chap. 4 - Chemical Reactions
... 2. Solid calcium reacts with oxygen gas. 3. Solutions of aluminum chloride & sodium carbonate are mixed. 4. Liquid magnesium bromide is decomposed at high temperature. 5. Solid nickel is reacted with aqueous magnesium sulfate. 6. Chlorine gas is reacted with aqueous potassium bromide. 7. Solid magne ...
... 2. Solid calcium reacts with oxygen gas. 3. Solutions of aluminum chloride & sodium carbonate are mixed. 4. Liquid magnesium bromide is decomposed at high temperature. 5. Solid nickel is reacted with aqueous magnesium sulfate. 6. Chlorine gas is reacted with aqueous potassium bromide. 7. Solid magne ...
DEPARTMENT OF CHEMISTRY LECTURE NOTES
... Equilibrium potential: When a metal electrode is dipped in a solution, if the rate of formation of ions is equal to the rate of their discharge, the electrode is said to be in equilibrium. The potential acquired by the electrode under such a condition is called the reversible or equilibrium pote ...
... Equilibrium potential: When a metal electrode is dipped in a solution, if the rate of formation of ions is equal to the rate of their discharge, the electrode is said to be in equilibrium. The potential acquired by the electrode under such a condition is called the reversible or equilibrium pote ...
Chemicals and Their Reactions
... Chemical formulas of substances involved The ratio of substances involved State of substances involved ...
... Chemical formulas of substances involved The ratio of substances involved State of substances involved ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.