system = part of the universe that contains the reaction or process
... phase changes • Heat is exchanged between the system and surroundings ...
... phase changes • Heat is exchanged between the system and surroundings ...
CHEM102 Chemistry II Spring 10-11 Mid
... 2) The balanced equation for the reaction occurring when iron(III) oxide, a solid, is reduced with pure carbon to produce carbon dioxide and molten iron is 2) C A) 2 FeO3 + 3 C (s) → 2 Fe (l) + 3 CO2 (g). B) 2 FeO + C (s) →? 2 Fe (l) + CO2 (g). C) 2 Fe2O3 + 3 C (s) → 4 Fe (l) + 3 CO2 (g). D) 4 Fe2O3 ...
... 2) The balanced equation for the reaction occurring when iron(III) oxide, a solid, is reduced with pure carbon to produce carbon dioxide and molten iron is 2) C A) 2 FeO3 + 3 C (s) → 2 Fe (l) + 3 CO2 (g). B) 2 FeO + C (s) →? 2 Fe (l) + CO2 (g). C) 2 Fe2O3 + 3 C (s) → 4 Fe (l) + 3 CO2 (g). D) 4 Fe2O3 ...
Atoms, Ions, and Molecules File
... Atoms of different elements are different. • Atoms are not changed into different atoms in a chemical reaction. • Compounds are formed when atoms of two or more elements combine. ...
... Atoms of different elements are different. • Atoms are not changed into different atoms in a chemical reaction. • Compounds are formed when atoms of two or more elements combine. ...
AP Chem Mr. Dehne Name: ___________ Date: Per#: ___ AP
... 5. Suppose two 200.0L tanks are to be filled separately with the gases helium and hydrogen. What mass of each gas is needed to produce a pressure of 135atm in its respective tank at 24 oC? 6. A 2.50L container is filled with 175g argon. a. If the pressure is 10.0atm, what is the temperature? b. If t ...
... 5. Suppose two 200.0L tanks are to be filled separately with the gases helium and hydrogen. What mass of each gas is needed to produce a pressure of 135atm in its respective tank at 24 oC? 6. A 2.50L container is filled with 175g argon. a. If the pressure is 10.0atm, what is the temperature? b. If t ...
Battery Materials
... An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy ...
... An electrical battery is one or more electrochemical cells that convert stored chemical energy into electrical energy ...
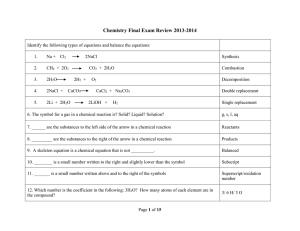
FINAL EXAM REVIEW PROBLEMS
... a. Suppose 6.71 x 103 g of titanium (IV) chloride is reacted with 2.45 x 10 3 g of oxygen. Calculate the maximum mass of titanium (IV) oxide that can form. b. If the percent yield of TiO2 is 75%, what mass was actually produced? ...
... a. Suppose 6.71 x 103 g of titanium (IV) chloride is reacted with 2.45 x 10 3 g of oxygen. Calculate the maximum mass of titanium (IV) oxide that can form. b. If the percent yield of TiO2 is 75%, what mass was actually produced? ...
SAMPLE QUESTION PAPER CHEMISTRY (043) CLASS XII (2013-14)
... (b) Metals of very high purity can be obtained by this method. The impure metal rod is mounted horizontally and heated by a circular electric heater at one end in an atmosphere of inert gas to form a thin molten zone. By slowly moving the heater, the molten zone is moved from one end of the rod to t ...
... (b) Metals of very high purity can be obtained by this method. The impure metal rod is mounted horizontally and heated by a circular electric heater at one end in an atmosphere of inert gas to form a thin molten zone. By slowly moving the heater, the molten zone is moved from one end of the rod to t ...
Mole Equation Homework Hint: Start equations with the numbers
... Hint: Start equations with the numbers given, and pay close attention to what the question is asking you to find. Usually, the first step in most stoichiometry problems (calculation of quantities in chemical equations) is to convert the given numbers to moles. SHOW YOUR WORK!!!!!!!!!!!!!!!!!!!!!!!!! ...
... Hint: Start equations with the numbers given, and pay close attention to what the question is asking you to find. Usually, the first step in most stoichiometry problems (calculation of quantities in chemical equations) is to convert the given numbers to moles. SHOW YOUR WORK!!!!!!!!!!!!!!!!!!!!!!!!! ...
+ H 2 O(l)
... AgNO3 (aq) + NaCl (aq) AgCl (s) + NaNO3 (aq) HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) NH4Cl (aq) + NaOH (aq) NH3 (g) + H2O (l) + NaCl (aq) Blue color for the products represents the driving force which allows the chemical reaction to occur. ...
... AgNO3 (aq) + NaCl (aq) AgCl (s) + NaNO3 (aq) HCl (aq) + NaOH (aq) NaCl (aq) + H2O (l) NH4Cl (aq) + NaOH (aq) NH3 (g) + H2O (l) + NaCl (aq) Blue color for the products represents the driving force which allows the chemical reaction to occur. ...
7.2: Properties, Names, and Formulas page 268 •Acids and bases
... 7.2: Properties, Names, and Formulas ...
... 7.2: Properties, Names, and Formulas ...
+ H 2 O(g)
... calculated and check if any of them changed. None of the oxidation numbers changes. CaO is a basic oxide as well as NH4+ is the protonated ammonia, then it can deliver H+ as an acid, then this reaction might be an acid base reaction. It occurs in heterogeneous phases, mostly in the solid phase with ...
... calculated and check if any of them changed. None of the oxidation numbers changes. CaO is a basic oxide as well as NH4+ is the protonated ammonia, then it can deliver H+ as an acid, then this reaction might be an acid base reaction. It occurs in heterogeneous phases, mostly in the solid phase with ...
A new and Economical Electrolytic Process
... process is operated at high temperatures (400-450 °C). The process is limited to 6080% single pass conversions and also results in the production of a mole of water for every mole of chlorine. There have been three commercial chlorine recycle processes based on reaction 2 but using different catalys ...
... process is operated at high temperatures (400-450 °C). The process is limited to 6080% single pass conversions and also results in the production of a mole of water for every mole of chlorine. There have been three commercial chlorine recycle processes based on reaction 2 but using different catalys ...
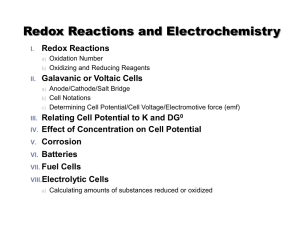
Redox Reactions
... 15e4e4FeS2 + 15O2 Fe(OH)3 + SO424FeS2 + 15O2 4Fe(OH)3 + 8SO424FeS2 + 15O2 +14H2O 4Fe(OH)3 + 8SO42- + 16H+ This reaction is the main cause of acid generation in drainage from sulfide ore deposits. Note that we get 4 moles of H+ for every mole of pyrite oxidized! ...
... 15e4e4FeS2 + 15O2 Fe(OH)3 + SO424FeS2 + 15O2 4Fe(OH)3 + 8SO424FeS2 + 15O2 +14H2O 4Fe(OH)3 + 8SO42- + 16H+ This reaction is the main cause of acid generation in drainage from sulfide ore deposits. Note that we get 4 moles of H+ for every mole of pyrite oxidized! ...
Lab Stuff
... 4. Compounds can be isomers if they have the same molecular formula, but different structural formulas. 5. Hydrocarbons can be evaluated as possible fuel sources by examining their heats of combustion. 6. Energy values can be inserted into a balanced chemical equation. 7. The specific heat of a subs ...
... 4. Compounds can be isomers if they have the same molecular formula, but different structural formulas. 5. Hydrocarbons can be evaluated as possible fuel sources by examining their heats of combustion. 6. Energy values can be inserted into a balanced chemical equation. 7. The specific heat of a subs ...
Lab Stuff:
... 4. Compounds can be isomers if they have the same molecular formula, but different structural formulas. 5. Hydrocarbons can be evaluated as possible fuel sources by examining their heats of combustion. 6. Energy values can be inserted into a balanced chemical equation. 7. The specific heat of a subs ...
... 4. Compounds can be isomers if they have the same molecular formula, but different structural formulas. 5. Hydrocarbons can be evaluated as possible fuel sources by examining their heats of combustion. 6. Energy values can be inserted into a balanced chemical equation. 7. The specific heat of a subs ...
Chem 101 notes review
... numbers of moles. 1 mol of C5H12 reacts with 8 mol of O2 to produce 5 mol of CO2, 6 mol of H2O, and releasing 3523 kJ is referred to as one mole of reactions. ...
... numbers of moles. 1 mol of C5H12 reacts with 8 mol of O2 to produce 5 mol of CO2, 6 mol of H2O, and releasing 3523 kJ is referred to as one mole of reactions. ...
Chemistry 20 Lesson 36 – The Whole Enchilada
... A portable hydrogen generator uses the reaction of calcium hydride and water to form calcium hydroxide and hydrogen. What volume of hydrogen at 96.5 kPa and 22.0°C can be produce from a 50.0 g cartridge of CaH2 (s)? ...
... A portable hydrogen generator uses the reaction of calcium hydride and water to form calcium hydroxide and hydrogen. What volume of hydrogen at 96.5 kPa and 22.0°C can be produce from a 50.0 g cartridge of CaH2 (s)? ...
Quiz Review Name Period 1. What is the equation that
... 15. When a substance condenses, the particles starts to slow down / speed up. 16. When a substance undergoes sublimation, it gets pulled together / separated. 17. Which of the following substance will have the lowest temperature after an hour of being in the sun? Water (4.184 J/goC) ...
... 15. When a substance condenses, the particles starts to slow down / speed up. 16. When a substance undergoes sublimation, it gets pulled together / separated. 17. Which of the following substance will have the lowest temperature after an hour of being in the sun? Water (4.184 J/goC) ...
Water Powerpoint
... • Water is a polar molecule • Oxygen atom has a stronger pull on e…takes on – charge. • Hydrogen atoms are weaker! Take on + charge. Water dissolves MANY compounds in the body allowing for metabolism to occur! ...
... • Water is a polar molecule • Oxygen atom has a stronger pull on e…takes on – charge. • Hydrogen atoms are weaker! Take on + charge. Water dissolves MANY compounds in the body allowing for metabolism to occur! ...
Chapter 4
... An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. ...
... An electrolyte is a substance that, when dissolved in water, results in a solution that can conduct electricity. A nonelectrolyte is a substance that, when dissolved, results in a solution that does not conduct electricity. ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.