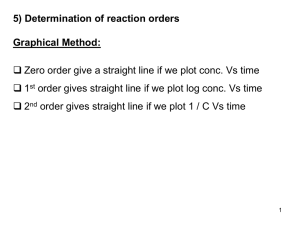
Scientific Principles: Chemical Properties
... you see that it takes one atom of sodium to combine with one item of chlorine • Stoichiometry aids us in determining the amounts of substances needed to fulfill the requirements of the reaction • Stoichiometry tells us that if you have 100 atoms of sodium and only one atom of chlorine you can only m ...
... you see that it takes one atom of sodium to combine with one item of chlorine • Stoichiometry aids us in determining the amounts of substances needed to fulfill the requirements of the reaction • Stoichiometry tells us that if you have 100 atoms of sodium and only one atom of chlorine you can only m ...
5 - BrainMass
... a. Is heat absorbed or evolved in the course of this reaction? b. Calculate the amount of heat transferred when 45.0 g of CH3OH (g) is decomposed by this reaction at constant pressure. c. For a given sample of CH3OH, the enthalpy change on reaction is 18.5 kJ. How many grams of hydrogen gas are prod ...
... a. Is heat absorbed or evolved in the course of this reaction? b. Calculate the amount of heat transferred when 45.0 g of CH3OH (g) is decomposed by this reaction at constant pressure. c. For a given sample of CH3OH, the enthalpy change on reaction is 18.5 kJ. How many grams of hydrogen gas are prod ...
Reacting Mass calculations
... calcium carbonate in the presence of oxygen. What mass of calcium carbonate is needed to remove 1 tonne of sulphur dioxide? 2 CaCO3 + 2 SO2 + O2 2 CaSO4 + 2 CO2 ...
... calcium carbonate in the presence of oxygen. What mass of calcium carbonate is needed to remove 1 tonne of sulphur dioxide? 2 CaCO3 + 2 SO2 + O2 2 CaSO4 + 2 CO2 ...
PHYSICAL SETTING CHEMISTRY
... from the cell diagram. [1] 71 Identify one metal from the passage that is more active than carbon and one metal from the passage that is less active than carbon. [1] 72 Write a balanced half-reaction equation for the reduction of the iron ions in iron(III) oxide to iron atoms. [1] ...
... from the cell diagram. [1] 71 Identify one metal from the passage that is more active than carbon and one metal from the passage that is less active than carbon. [1] 72 Write a balanced half-reaction equation for the reduction of the iron ions in iron(III) oxide to iron atoms. [1] ...
Honors Chapter 11 Reactions
... Write Word equations to help you organize reactants and products Be sure to include symbols showing states of each reactant and product Be sure to write the correct formula ...
... Write Word equations to help you organize reactants and products Be sure to include symbols showing states of each reactant and product Be sure to write the correct formula ...
Chapter 4 Reactions in Aqueous Solutions
... Predict whether each of the following will occur. For the reactions that do occur, write a balanced net ionic reaction for each. - Copper metal is placed into a solution of silver nitrate Cu (s) ...
... Predict whether each of the following will occur. For the reactions that do occur, write a balanced net ionic reaction for each. - Copper metal is placed into a solution of silver nitrate Cu (s) ...
Chemistry Unit Test Review
... Which is not a common physical property of Fe, Co, Ni, Cu, and Zn? ...
... Which is not a common physical property of Fe, Co, Ni, Cu, and Zn? ...
CHEM%1212K% Final%Exam% Summer%2011% K
... B)%The%pH%of%the%solution%is%neutral%because%it%was%made%from%a%strong%base% ...
... B)%The%pH%of%the%solution%is%neutral%because%it%was%made%from%a%strong%base% ...
Name
... Water has a density of 1.00 g/cm3. The average adult has a density of 0.97 g/cm3. The 0.97 value means that in fresh water, most people float about 97% underwater and 3% above water. It’s a little easier to float in saltwater with 95% under the surface and 5% above water. In either case, it doesn’t ...
... Water has a density of 1.00 g/cm3. The average adult has a density of 0.97 g/cm3. The 0.97 value means that in fresh water, most people float about 97% underwater and 3% above water. It’s a little easier to float in saltwater with 95% under the surface and 5% above water. In either case, it doesn’t ...
Unit 12 Worksheet Answers
... 16. A solution is made by dissolving 17.1 g of sucrose, C 12 H 22 O 11 in 275 g of water. a. What is the solute? Sucrose b. What is the solvent? water c. What is the molality? 0.182 m 17. What is the van’t hoff factor (how many ions does it turn into) for each of the following? ...
... 16. A solution is made by dissolving 17.1 g of sucrose, C 12 H 22 O 11 in 275 g of water. a. What is the solute? Sucrose b. What is the solvent? water c. What is the molality? 0.182 m 17. What is the van’t hoff factor (how many ions does it turn into) for each of the following? ...
eastern illinois university
... 22. Consider the following unbalanced equation: LaCl3 + Na2CO3 La2(CO3)3 + NaCl. When this equation is balanced (simplest whole number coefficients), the coefficient for NaCl is: a. 1 b. 2 c. 3 d. 5 e. 6 23. Consider the balanced, but incomplete, equation: 2AlCl3 + Ca3N22X + 3CaCl2. The formula o ...
... 22. Consider the following unbalanced equation: LaCl3 + Na2CO3 La2(CO3)3 + NaCl. When this equation is balanced (simplest whole number coefficients), the coefficient for NaCl is: a. 1 b. 2 c. 3 d. 5 e. 6 23. Consider the balanced, but incomplete, equation: 2AlCl3 + Ca3N22X + 3CaCl2. The formula o ...
Chemical Reactions
... • Higher Temperatures = faster reaction • Particles speed up or slow down…speeding up or slowing down the reaction… ...
... • Higher Temperatures = faster reaction • Particles speed up or slow down…speeding up or slowing down the reaction… ...
Examples
... reacts with bromide in an acidic solution Permanganate reacts with oxalate in an acidic solution Calcium metal reacts with permanganate in a sodium hydroxide solution ...
... reacts with bromide in an acidic solution Permanganate reacts with oxalate in an acidic solution Calcium metal reacts with permanganate in a sodium hydroxide solution ...
Chapter 8 Thermochemistry: Chemical Energy
... N2H4, and dinitrogen tetroxide, N2O4. These compounds react to give gaseous nitrogen, N2 and water vapor, evolving 1049 kJ of heat at constant pressure when 1 mol N2O4 reacts. Write the thermochemical equation for this reaction Write the thermochemical equation for the reverse of the reaction How mu ...
... N2H4, and dinitrogen tetroxide, N2O4. These compounds react to give gaseous nitrogen, N2 and water vapor, evolving 1049 kJ of heat at constant pressure when 1 mol N2O4 reacts. Write the thermochemical equation for this reaction Write the thermochemical equation for the reverse of the reaction How mu ...
(1) Dissolves, accompanied by evolution of flammable gas (2
... (a) The second ionization energy of sodium is about three times greater than the second ionization energy of magnesium. (b) The difference between the atomic radii of Na and K is relatively large compared to the difference between the atomic radii of Rb and Cs. (c) A sample of solid nickel chloride ...
... (a) The second ionization energy of sodium is about three times greater than the second ionization energy of magnesium. (b) The difference between the atomic radii of Na and K is relatively large compared to the difference between the atomic radii of Rb and Cs. (c) A sample of solid nickel chloride ...
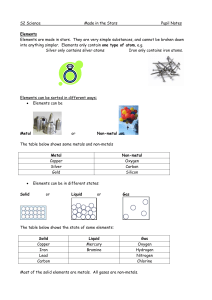
Made in the Stars Notes
... Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graphite, a form of carbon, which is a good conductor. ...
... Non-metal solids are usually brittle (they break easily). Non-metals can be solids, liquids or gases at room temperature. Non-metals usually have low melting and boiling points. They are poor conductors of electricity. The exception is graphite, a form of carbon, which is a good conductor. ...
Chemical Reactions
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
The s-Block Elements - GCG-42
... BeCl2 is essentially covalent, with comparatively low m.pt. The lower members in group II form essentially ionic chlorides, with Mg having intermediate properties. ...
... BeCl2 is essentially covalent, with comparatively low m.pt. The lower members in group II form essentially ionic chlorides, with Mg having intermediate properties. ...
Review Unit - hrsbstaff.ednet.ns.ca
... CH4 + 2O2 → CO2 + 2H2O + energy The release of energy is shown as a product in the equation. We can show this in an energy level diagram: ...
... CH4 + 2O2 → CO2 + 2H2O + energy The release of energy is shown as a product in the equation. We can show this in an energy level diagram: ...
Practice Questions Section 2
... Write balanced chemical equations for each of the following. Pay close attention to the physical states! Also - you must include the charge when writing ions, otherwise your answer is incorrect. Do not balance these equations using fractions for coefficients. sulfur dioxide gas combines with oxygen ...
... Write balanced chemical equations for each of the following. Pay close attention to the physical states! Also - you must include the charge when writing ions, otherwise your answer is incorrect. Do not balance these equations using fractions for coefficients. sulfur dioxide gas combines with oxygen ...
water properties - What is Chemistry
... action of water as a reagent. Esters and polysaccharides are two classes of biologically important organic compounds that undergo hydrolysis; starch is a polysaccharide, and its hydrolysis is a step in digestion of many important foods; triglycerides are esters involved in fat metabolism, with hydro ...
... action of water as a reagent. Esters and polysaccharides are two classes of biologically important organic compounds that undergo hydrolysis; starch is a polysaccharide, and its hydrolysis is a step in digestion of many important foods; triglycerides are esters involved in fat metabolism, with hydro ...
Electrolysis of water
Electrolysis of water is the decomposition of water (H2O) into oxygen (O2) and hydrogen gas (H2) due to an electric current being passed through the water.This technique can be used to make hydrogen fuel (hydrogen gas) and breathable oxygen; though currently most industrial methods make hydrogen fuel from natural gas instead.