Date: 16 / 01 / 2014 - Qatar University QSpace
... Figure 4.2 Adsorption isotherm of Cu-ZSM5 catalyst ...................................................... 51 Figure 4.3 Illustration of how SEM works...................................................................... 52 Figure 4.4 SEM scan of Cu-ZSM5............................................... ...
... Figure 4.2 Adsorption isotherm of Cu-ZSM5 catalyst ...................................................... 51 Figure 4.3 Illustration of how SEM works...................................................................... 52 Figure 4.4 SEM scan of Cu-ZSM5............................................... ...
An Analogy for an Equilibrium Reaction
... a) addition of ammonia to use up the extra ammonia the system will shift to the left b) removal of NO2 to replace some of the nitrogen dioxide the system will shift to the left c) removal of water vapour to replace some of the water vapour the system will shift to the right d) addition of hydrogen t ...
... a) addition of ammonia to use up the extra ammonia the system will shift to the left b) removal of NO2 to replace some of the nitrogen dioxide the system will shift to the left c) removal of water vapour to replace some of the water vapour the system will shift to the right d) addition of hydrogen t ...
From Kinetics to Equilibrium
... (The gas inflates the bag.) This chemical reaction occurs almost instantaneously. It inflates the air bag quickly enough to cushion a driver’s impact. On the other hand, the reaction of iron with oxygen to form rust proceeds quite slowly. Most Canadians know that the combination of road salt and wet ...
... (The gas inflates the bag.) This chemical reaction occurs almost instantaneously. It inflates the air bag quickly enough to cushion a driver’s impact. On the other hand, the reaction of iron with oxygen to form rust proceeds quite slowly. Most Canadians know that the combination of road salt and wet ...
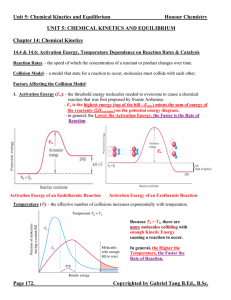
Unit 5: Chemical Kinetics and Equilibrium
... at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than reactants at the state of equilibrium. ([A]eq and [B]eq or PA, eq and PB, eq >> [C]eq and [D]eq or PC ...
... at the state of equilibrium. ([C]eq and [D]eq or PC, eq and PD, eq >> [A]eq and [B]eq or PA, eq and PB, eq) 2. When K << 1, the equilibrium system favours the reactants. There are less products than reactants at the state of equilibrium. ([A]eq and [B]eq or PA, eq and PB, eq >> [C]eq and [D]eq or PC ...
Chapter 4 Aqueous Reactions and Solution Stoichiometry
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
document
... C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) Calculate the number of moles of oxygen required to react exactly with 4.3 moles of propane, C3H8, in the above reaction 4.3 moles of C3H8 requires how many moles of O2 There is a 1:5 ratio So 4.3(1) : 4.3(5) ...
... C3H8(g) + 5O2(g) → 3CO2(g) + 4H2O(g) Calculate the number of moles of oxygen required to react exactly with 4.3 moles of propane, C3H8, in the above reaction 4.3 moles of C3H8 requires how many moles of O2 There is a 1:5 ratio So 4.3(1) : 4.3(5) ...
CHAPTER 9 Notes
... c. When 2.50 g of K and 1.00 g Cl2 react together, the mass of KCl produced is _____2.10 g_______________, the limiting reactant is____Cl2____________, and the reactant in excess is _______K__________________. ...
... c. When 2.50 g of K and 1.00 g Cl2 react together, the mass of KCl produced is _____2.10 g_______________, the limiting reactant is____Cl2____________, and the reactant in excess is _______K__________________. ...
Review Packet Answers - Bremerton School District
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
Calculations with Chemical Formulas and Equations
... 3NaHCO3(aq) + H3C6H5O7(aq) ------> 3CO2(g) + 3H2O(l) + Na3C6H5O7(aq) 1.0g 1.0g 84g/mol 192g/mol 44g/mol 1.0g(1mol/84g) 1.0(1mol/192g) 0.012 mol 0.0052 mol 0.0052(3)=0.016 0.0052 mol (if citrate limiting) 0.012 mol 0.012(1/3)=.0040mol 0.012 moles CO2 44g/mol(0.012mol)=0.53g CO2 .0052-.0040=.0012 left ...
... 3NaHCO3(aq) + H3C6H5O7(aq) ------> 3CO2(g) + 3H2O(l) + Na3C6H5O7(aq) 1.0g 1.0g 84g/mol 192g/mol 44g/mol 1.0g(1mol/84g) 1.0(1mol/192g) 0.012 mol 0.0052 mol 0.0052(3)=0.016 0.0052 mol (if citrate limiting) 0.012 mol 0.012(1/3)=.0040mol 0.012 moles CO2 44g/mol(0.012mol)=0.53g CO2 .0052-.0040=.0012 left ...
Unit 3: 1 Equilibrium and the Constant, K
... environmental processes that are reversible, construct an explanation that connects the observations to the reversibility of the underlying chemical reactions or processes. [See SP 6.2; Essential knowledge 6.A.1] Learning objective 6.2 The student can, given a manipulation of a chemical reaction or ...
... environmental processes that are reversible, construct an explanation that connects the observations to the reversibility of the underlying chemical reactions or processes. [See SP 6.2; Essential knowledge 6.A.1] Learning objective 6.2 The student can, given a manipulation of a chemical reaction or ...
Catalytic decomposition of N2O over Rh/Zn–Al2O3 catalysts
... lead to more active catalysts due to the improved dispersion of Rh species.28 Parres-Esclapez et al. found that Sr can promote the activity of Rh/Al2O3 due to the improved dispersion and reducibility of Rh species.29 Zhao et al. reported that Rh/SiO2–Al2O3 shows high activity, because oxygen desorpt ...
... lead to more active catalysts due to the improved dispersion of Rh species.28 Parres-Esclapez et al. found that Sr can promote the activity of Rh/Al2O3 due to the improved dispersion and reducibility of Rh species.29 Zhao et al. reported that Rh/SiO2–Al2O3 shows high activity, because oxygen desorpt ...
A* PLC Legacy GCSE Chemistry (all boards)
... The equation shows that the zinc atoms (metal) have formed positively charged zinc ions, i.e., they have lost electrons. This means the zinc atoms have been oxidised. • The positively charged copper ions have formed copper atoms, i.e., they have gained the electrons which the zinc atoms have lost. T ...
... The equation shows that the zinc atoms (metal) have formed positively charged zinc ions, i.e., they have lost electrons. This means the zinc atoms have been oxidised. • The positively charged copper ions have formed copper atoms, i.e., they have gained the electrons which the zinc atoms have lost. T ...
aq - Haverford Alchemy
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
... the ions that each contains. We then correlate these charged ionic species with the ones shown in the diagram. Solve: The diagram shows twice as many cations as anions, consistent with the formulation K 2SO4. Aqueous Check: Notice that the total net charge in the diagram is zero, as it must be if it ...
A Model For the Calculation of Solvent ... Reaction Rates for Process Design Purposes
... models for the assessment of solvents as reaction media that are fast, and therefore able to screen large numbers of molecules in a reasonable time, as well as applicable to a wide range of solvents and reactions and able to provide results that are accurate for process design needs. Solvent effects ...
... models for the assessment of solvents as reaction media that are fast, and therefore able to screen large numbers of molecules in a reasonable time, as well as applicable to a wide range of solvents and reactions and able to provide results that are accurate for process design needs. Solvent effects ...
chemistry - The Aga Khan University
... Trends in Density 13.2.2 Trends in Reactivity with Water 13.2.3 Reactions with Oxygen 13.2.3.1 Reactions with Air or Oxygen and the formation of Normal Oxides, Peroxides, Super Oxides and their Stability 13.2.3.2 Reactions of Oxides with Water and Dilute Acids 13.2.4 Reactions with Chlorine 13.2.5 E ...
... Trends in Density 13.2.2 Trends in Reactivity with Water 13.2.3 Reactions with Oxygen 13.2.3.1 Reactions with Air or Oxygen and the formation of Normal Oxides, Peroxides, Super Oxides and their Stability 13.2.3.2 Reactions of Oxides with Water and Dilute Acids 13.2.4 Reactions with Chlorine 13.2.5 E ...
LaBrake, Fundamentals Diagnostic Questions
... c) iodine, I d) oxygen, O e) potassium, K 40. Which of the following common names is not correctly matched with the formula? a) NH3, ammonia b) H2O, water c) C2H2, dicarbon dihyrdide (correct) d) C2H4, ethylene e) N2O, nitrous oxide 41. Which has more molecules: a mole of H2O or a mole of O2? a) a m ...
... c) iodine, I d) oxygen, O e) potassium, K 40. Which of the following common names is not correctly matched with the formula? a) NH3, ammonia b) H2O, water c) C2H2, dicarbon dihyrdide (correct) d) C2H4, ethylene e) N2O, nitrous oxide 41. Which has more molecules: a mole of H2O or a mole of O2? a) a m ...
Homework extension
... Suggested Answers Additional Guidance Marks awarded for this answer will be determined by the Quality of Written Communication (QWC) as well as the standard of the scientific response. Teachers should and apply a ‘bestfit’ approach to the marking. 0 marks Level 1 (1-2 marks) Level 2 (3-4 marks) Leve ...
... Suggested Answers Additional Guidance Marks awarded for this answer will be determined by the Quality of Written Communication (QWC) as well as the standard of the scientific response. Teachers should and apply a ‘bestfit’ approach to the marking. 0 marks Level 1 (1-2 marks) Level 2 (3-4 marks) Leve ...
department of pure and applied chemistry
... The course is designed to impart the communication skills in the medium of English. Emphasis is on introducing students to English for Academic purposes and specifically focuses on: Study skills and Study plans; Listening and Note taking; Speaking (sounds of English, stress, intonation and rhythm); ...
... The course is designed to impart the communication skills in the medium of English. Emphasis is on introducing students to English for Academic purposes and specifically focuses on: Study skills and Study plans; Listening and Note taking; Speaking (sounds of English, stress, intonation and rhythm); ...
Chapter 1: Chemistry: The Study of Change
... percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of P and 68.0 g of I2 react according to the following chemical equation? (Section: 3.9) ...
... percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of P and 68.0 g of I2 react according to the following chemical equation? (Section: 3.9) ...