Chapter 1: Chemistry: The Study of Change
... percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of P and 68.0 g of I2 react according to the following chemical equation? (Section: 3.9) ...
... percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. What is the limiting reagent when 27.0 g of P and 68.0 g of I2 react according to the following chemical equation? (Section: 3.9) ...
Mole Concept
... will require Y mol A x (b mol B / a mol A) = Yb/a mol B, since the stoichiometric coefficients are conversion factors between different species. We define 1 mol of this reaction as the process of mixing a mol of A with b mol of B to form c mol C and d mol D. Thus, if we have Y mol of A, we can conve ...
... will require Y mol A x (b mol B / a mol A) = Yb/a mol B, since the stoichiometric coefficients are conversion factors between different species. We define 1 mol of this reaction as the process of mixing a mol of A with b mol of B to form c mol C and d mol D. Thus, if we have Y mol of A, we can conve ...
Chapter 3
... In Chapter 2, we saw the importance of relative numbers of atoms in the formation of compounds. We also learned how relative masses of atoms can be based on the arbitrary choice of the carbon-12 atom as a standard (Section 2.4). Now, we introduce a concept that enables us to deal with actual rather ...
... In Chapter 2, we saw the importance of relative numbers of atoms in the formation of compounds. We also learned how relative masses of atoms can be based on the arbitrary choice of the carbon-12 atom as a standard (Section 2.4). Now, we introduce a concept that enables us to deal with actual rather ...
Chapter 16
... phase equilibrium can be rather involved. The equilibrium criterion for reacting systems is based on the second law of thermodynamics; more specifically, the increase of entropy principle. For adiabatic systems, chemical equilibrium is established when the entropy of the reacting system reaches a ma ...
... phase equilibrium can be rather involved. The equilibrium criterion for reacting systems is based on the second law of thermodynamics; more specifically, the increase of entropy principle. For adiabatic systems, chemical equilibrium is established when the entropy of the reacting system reaches a ma ...
heterogeneous chiral catalyst derived from hydrolyzed
... cumbersome and expensive. However, following the understanding that different enantiomers may have qualitatively distinct physiological effects, [16-20] and instigated by stricter regulations from health authorities, a growing number of new drugs are now marketed as single enantiomers [21-23]. The t ...
... cumbersome and expensive. However, following the understanding that different enantiomers may have qualitatively distinct physiological effects, [16-20] and instigated by stricter regulations from health authorities, a growing number of new drugs are now marketed as single enantiomers [21-23]. The t ...
Exam Review Packet Table of Contents
... An incorrect statement in an otherwise correct 2 pt response will result in a score of 1 pt The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The ...
... An incorrect statement in an otherwise correct 2 pt response will result in a score of 1 pt The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The ...
PART 3-ICHO 11-15
... first quantitatively separated from the precipitate, and then hydrogen sulphide was passed through the separated solution to saturation. The resulting precipitate containing metal B was separated, washed and dried. The mass of the precipitate was 0.6613 g. The precipitate containing the compounds of ...
... first quantitatively separated from the precipitate, and then hydrogen sulphide was passed through the separated solution to saturation. The resulting precipitate containing metal B was separated, washed and dried. The mass of the precipitate was 0.6613 g. The precipitate containing the compounds of ...
Homework1-4-Answers
... Chapter 1: Chemistry: The Study of Change 22. Phosphorus reacts with iodine as shown in the chemical reaction below. What is the percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. W ...
... Chapter 1: Chemistry: The Study of Change 22. Phosphorus reacts with iodine as shown in the chemical reaction below. What is the percent yield of the reaction if 28.2 g PI3 is obtained from the reaction of 48.0 g of I2 with excess phosphorus? (Section: 3.10) 2P(s) + 3I2(s) 2PI3(s) Ans: 54.3% 23. W ...
Massachusetts Tests for Educator Licensure (MTEL )
... contain at least one of the cations shown in the reference table. Given the chromatogram shown above, the unknown consists of: A. ...
... contain at least one of the cations shown in the reference table. Given the chromatogram shown above, the unknown consists of: A. ...
Photo-oxidation of pinonaldehyde at low NOx
... literature (Glasius et al., 1997). Though we recently described SOA chemistry under highNOx conditions (Chacon-Madrid and Donahue, 2011), it is important to explore its chemistry in low-NOx conditions because products of biogenic species are concentrated in areas where NOx concentrations are low (At ...
... literature (Glasius et al., 1997). Though we recently described SOA chemistry under highNOx conditions (Chacon-Madrid and Donahue, 2011), it is important to explore its chemistry in low-NOx conditions because products of biogenic species are concentrated in areas where NOx concentrations are low (At ...
ANNEX (Manuscrits posteriors a la Comissió de Doctorat de Juliol del...
... Dimethyl substitution of [1]- at the 8, 8’ positions was achieved by B-C cross coupling reaction employing a modified Kumada reaction (Scheme 2). Addition of 5 equivalents of methylmagnesium bromide to a cooled (0°C) solution of Cs[2] in THF, followed by a catalytic amount of [PdCl2(PPh3)2] and CuI ...
... Dimethyl substitution of [1]- at the 8, 8’ positions was achieved by B-C cross coupling reaction employing a modified Kumada reaction (Scheme 2). Addition of 5 equivalents of methylmagnesium bromide to a cooled (0°C) solution of Cs[2] in THF, followed by a catalytic amount of [PdCl2(PPh3)2] and CuI ...
Oxygen Carriers Materials for Chemical
... Carbon dioxide is the gas which contributes most to the greenhouse effect. It is released in large quantities from fossil fuel-based power plants around the world. It is generally accepted that a rapid decrease in the emissions of carbon dioxide is needed. One method to achieve rapid reductions in t ...
... Carbon dioxide is the gas which contributes most to the greenhouse effect. It is released in large quantities from fossil fuel-based power plants around the world. It is generally accepted that a rapid decrease in the emissions of carbon dioxide is needed. One method to achieve rapid reductions in t ...
Model-based approach to plant-wide economic control of uid
... investigated disturbances showed considerable influence on the products composition. Taking into account the very high volume production of an industrial FCC unit, these disturbances can have a significant economic impact. The fresh feed coke formation factor is one of the most important disturbance ...
... investigated disturbances showed considerable influence on the products composition. Taking into account the very high volume production of an industrial FCC unit, these disturbances can have a significant economic impact. The fresh feed coke formation factor is one of the most important disturbance ...
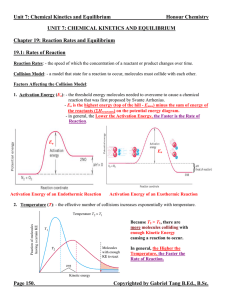
Unit 7 Reaction Rates and Equilibrium Notes
... Chemical Equilibrium: - the state at which the concentrations of all reactants and products remain constant with time (the Forward Reaction Rate = Reverse Reaction Rate). - the equilibrium state is dynamic (not static). Chemical species are continuously converting from reactants to products and vice ...
... Chemical Equilibrium: - the state at which the concentrations of all reactants and products remain constant with time (the Forward Reaction Rate = Reverse Reaction Rate). - the equilibrium state is dynamic (not static). Chemical species are continuously converting from reactants to products and vice ...
13 CHEMICAL EQUILIBRIUM W MODULE - 5
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...
... reaction, it is believed that all the reactants would be converted into products with the release or absorption of energy. This is not true in all cases. Many chemical reactions proceed only to a certain extent and stop. When analysed, the resulting mixture contains both the reactants and products. ...
2 - cloudfront.net
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
... How do you find out which is limited? 3. The chemical that makes the least amount of product is the “limiting reactant”. You can recognize limiting reactant problems because they will give you 2 amounts of chemical 4. Do two stoichiometry problems, one for each reactant given ...
Chapter 3:Mass Relationships in Chemical Reactions
... trying to balance an equation, this means subscripts. • Start with the most complex formula first. • Balance polyatomic ions as a single unit unless they breakdown. • The coefficients must be whole numbers. • After balancing an equation, check each symbol with its corresponding number. • Finally, Ma ...
... trying to balance an equation, this means subscripts. • Start with the most complex formula first. • Balance polyatomic ions as a single unit unless they breakdown. • The coefficients must be whole numbers. • After balancing an equation, check each symbol with its corresponding number. • Finally, Ma ...
EQUILIBRIUM
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
EQUILIBRIUM
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
... (A complete explanation based on Le Chatelier's principle is also acceptable.) c) two points The mass of NH4HS increases. A decrease in volume causes the pressure of each gas to increase. To maintain the value of the pressure equilibrium constant, Kp, the pressure of each of the gases must decrease. ...
Chapter - WTPS.org
... • the most abundant elements of the Earth’s crust are O and Si • silicates are covalent atomic solids of Si and O and minor amounts of other elements found in rocks, soils, and clays silicates have variable structures – leading to the variety of properties found in rocks, clays, and soils Tro, Ch ...
... • the most abundant elements of the Earth’s crust are O and Si • silicates are covalent atomic solids of Si and O and minor amounts of other elements found in rocks, soils, and clays silicates have variable structures – leading to the variety of properties found in rocks, clays, and soils Tro, Ch ...
Chapter22_LEC
... • the most abundant elements of the Earth’s crust are O and Si • silicates are covalent atomic solids of Si and O and minor amounts of other elements found in rocks, soils, and clays silicates have variable structures – leading to the variety of properties found in rocks, clays, and soils Tro, Ch ...
... • the most abundant elements of the Earth’s crust are O and Si • silicates are covalent atomic solids of Si and O and minor amounts of other elements found in rocks, soils, and clays silicates have variable structures – leading to the variety of properties found in rocks, clays, and soils Tro, Ch ...