431 KB / 47 pages
... second would be the anode where Zn is oxidized to Zn2+. The net effect would be no plating of the zinc, so the meter would be useless (as well as pretty inconvenient). Problem 10.9. We look at each of these unbalanced reactions to see which species on the left is oxidized (the anode reaction) and wh ...
... second would be the anode where Zn is oxidized to Zn2+. The net effect would be no plating of the zinc, so the meter would be useless (as well as pretty inconvenient). Problem 10.9. We look at each of these unbalanced reactions to see which species on the left is oxidized (the anode reaction) and wh ...
Chemical Equilibrium
... However, this is not the case. The reverse chemical reaction is also taking place: 2HI → H2 + I2 It acts to undo what the first reaction does. Eventually, the reverse reaction proceeds so quickly that it matches the speed of the forward reaction. When that happens, any continued overall reaction stop ...
... However, this is not the case. The reverse chemical reaction is also taking place: 2HI → H2 + I2 It acts to undo what the first reaction does. Eventually, the reverse reaction proceeds so quickly that it matches the speed of the forward reaction. When that happens, any continued overall reaction stop ...
Surface and sub-surface reactions during low temperature
... (PP) films lacking reactive functional groups show smaller mass uptake during TMA exposure, similar to that measured during Al2O3 ALD on dense Al2O3 substrates. The mass uptake observed on PP, however, is more sensitive to deposition temperature than the other polymers investigated. In situ infrared ...
... (PP) films lacking reactive functional groups show smaller mass uptake during TMA exposure, similar to that measured during Al2O3 ALD on dense Al2O3 substrates. The mass uptake observed on PP, however, is more sensitive to deposition temperature than the other polymers investigated. In situ infrared ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... Chemistry: A Molecular Approach, 1st Ed. Nivaldo Tro ...
... Chemistry: A Molecular Approach, 1st Ed. Nivaldo Tro ...
Document
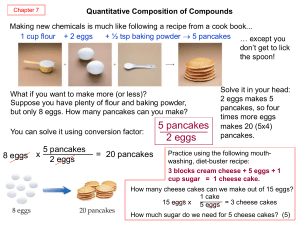
... liquid used in rocket engines has a molar mass of 92.0 g mole-1. What is the molecular formula of this substance? (N2O4) 2. A sample of a brown gas, a major air pollutant, is found to contain 2.34 g N and 5.34 g O. Determine an empirical formula for this substance. (NO2) 3. Percent composition of a ...
... liquid used in rocket engines has a molar mass of 92.0 g mole-1. What is the molecular formula of this substance? (N2O4) 2. A sample of a brown gas, a major air pollutant, is found to contain 2.34 g N and 5.34 g O. Determine an empirical formula for this substance. (NO2) 3. Percent composition of a ...
Ch16 - WordPress.com
... 2HI(g) H2(g) + I2(g) is 48.8 at 455°C. An equilibrium mixture in a 2.0 L vessel at this temperature contains 0.220 mol of H2 and 0.110 mol of I2. a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium ...
... 2HI(g) H2(g) + I2(g) is 48.8 at 455°C. An equilibrium mixture in a 2.0 L vessel at this temperature contains 0.220 mol of H2 and 0.110 mol of I2. a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium ...
Michelle Tostado WRIT 340 26 February 2013 MWF 11:00 a.m.
... flavors and bold characteristics. While there is no single chemistry composition that identifies whisky, whiskies contain hundreds of compounds that give each whisky its distinguishing taste. These different compounds and essences within the whisky arise from the diminutive variances in the fundamen ...
... flavors and bold characteristics. While there is no single chemistry composition that identifies whisky, whiskies contain hundreds of compounds that give each whisky its distinguishing taste. These different compounds and essences within the whisky arise from the diminutive variances in the fundamen ...
Modifying the stereochemistry of an enzyme
... here is a growing demand in the chemical and biotechnology industries for reliable and efficient methods for the production of enantiomerically pure compounds. Increasingly, synthetic chemists are exploiting enzymes in the asymmetric and stereoselective synthesis of chiral building blocks (1). Howev ...
... here is a growing demand in the chemical and biotechnology industries for reliable and efficient methods for the production of enantiomerically pure compounds. Increasingly, synthetic chemists are exploiting enzymes in the asymmetric and stereoselective synthesis of chiral building blocks (1). Howev ...
Ch16
... 2HI(g) H2(g) + I2(g) is 48.8 at 455°C. An equilibrium mixture in a 2.0 L vessel at this temperature contains 0.220 mol of H2 and 0.110 mol of I2. a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium ...
... 2HI(g) H2(g) + I2(g) is 48.8 at 455°C. An equilibrium mixture in a 2.0 L vessel at this temperature contains 0.220 mol of H2 and 0.110 mol of I2. a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium ...
The First Law of Thermodynamics Does Not Predict Spontaneous
... • In the chemical reactions, fewer moles of crystalline solids produce more moles of gases and/or solvated ions, so once again, the freedom of motion of the particles increases and their energy of motion is more dispersed: less freedom of particle motion more freedom of particle motion localized e ...
... • In the chemical reactions, fewer moles of crystalline solids produce more moles of gases and/or solvated ions, so once again, the freedom of motion of the particles increases and their energy of motion is more dispersed: less freedom of particle motion more freedom of particle motion localized e ...
Chapter One Hemilabile Ligands in Transition
... In this very simple example, the enthalpy difference is within experimental error indicating the chelate effect can be traced entirely to the entropy difference between the change of ligand numbers.5 The main cause of the large entropy increase is related to the net number of unbound molecules or li ...
... In this very simple example, the enthalpy difference is within experimental error indicating the chelate effect can be traced entirely to the entropy difference between the change of ligand numbers.5 The main cause of the large entropy increase is related to the net number of unbound molecules or li ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... Chemistry: A Molecular Approach, 1st Ed. Nivaldo Tro ...
... Chemistry: A Molecular Approach, 1st Ed. Nivaldo Tro ...
Worked solutions to the problems
... your students need to show in Melbourne. We have tried to highlight the procedures in each exercise that need some particular caution, even for students of Olympiad level but our warnings cannot be comprehensive - your students will still need your careful supervision. We have also not included spec ...
... your students need to show in Melbourne. We have tried to highlight the procedures in each exercise that need some particular caution, even for students of Olympiad level but our warnings cannot be comprehensive - your students will still need your careful supervision. We have also not included spec ...
CHEMICAL EQUILIBRIUM
... 8. Even though the individual sets of equilibrium concentrations are quite different for the different situations, the equilibrium constant which depends on the ratio of the concentrations, remains the same. 9. Each set of equilibrium concentrations is called an ______________________________. 10. ...
... 8. Even though the individual sets of equilibrium concentrations are quite different for the different situations, the equilibrium constant which depends on the ratio of the concentrations, remains the same. 9. Each set of equilibrium concentrations is called an ______________________________. 10. ...
Chapter 1 – Reaction Kinetics Answer Key
... 101.3 kPa due to a higher elevation. The temperature difference would be much more significant than the pressure as we are very close to sea level. This means molar volume is likely a bit smaller than 24.5 L/mol (or 26.5 L/mol for trial two). Dividing by a smaller number would lead to a LARGER MASS ...
... 101.3 kPa due to a higher elevation. The temperature difference would be much more significant than the pressure as we are very close to sea level. This means molar volume is likely a bit smaller than 24.5 L/mol (or 26.5 L/mol for trial two). Dividing by a smaller number would lead to a LARGER MASS ...
CHemiStrY - Cabrillo College
... Chemistry is the study of the properties, composition and transformations of all material substances. It is often called the “central science” since it draws from mathematics and physics and forms a necessary background to the study of the earth sciences and all the biological disciplines, including ...
... Chemistry is the study of the properties, composition and transformations of all material substances. It is often called the “central science” since it draws from mathematics and physics and forms a necessary background to the study of the earth sciences and all the biological disciplines, including ...
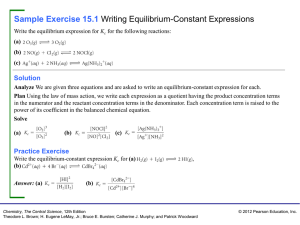
Sample Exercise 15.1 Writing Equilibrium
... Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occur. For the forward process to occur, there must be some calcium carbonate present. For the reverse process to occur, there must be both calcium oxide and carbon dioxide. In both cases ...
... Plan For equilibrium to be achieved, it must be possible for both the forward process and the reverse process to occur. For the forward process to occur, there must be some calcium carbonate present. For the reverse process to occur, there must be both calcium oxide and carbon dioxide. In both cases ...
quantitative_chemistry
... By choosing to weigh out a mass in grams that has the same numerical value as the formula mass in amu’s (180.2 grams and 180.2 amu, in the case of aspirin) we are able to ensure that the sample contains just as many molecules as there are amu’s in 1 g. This gives us a very useful automatic link betw ...
... By choosing to weigh out a mass in grams that has the same numerical value as the formula mass in amu’s (180.2 grams and 180.2 amu, in the case of aspirin) we are able to ensure that the sample contains just as many molecules as there are amu’s in 1 g. This gives us a very useful automatic link betw ...
Chapter 09 An Overview of Chemical Reactions Notes
... is assigned the mass of 12.00. This means that chlorine atom (with an atomic mass of 35.45) is roughly 1.5 times more massive than a carbon atom. Formula Mass: - the sum of all the atomic masses in a chemical formula. - it is the same as the molar mass (M), which is the mass per mole (6.02 × 1023 mo ...
... is assigned the mass of 12.00. This means that chlorine atom (with an atomic mass of 35.45) is roughly 1.5 times more massive than a carbon atom. Formula Mass: - the sum of all the atomic masses in a chemical formula. - it is the same as the molar mass (M), which is the mass per mole (6.02 × 1023 mo ...
BSc Chemistry Syllabus - St. Xavier`s College
... a. To learn preparations reactions and properties of alkane, alkene and alkynes. b. To learn estimation of nitrogen and molecular weight determination of organic acids and bases c. To learn basics of selected organic reactions and mechanisms d. To understand the atomic structure e. To learn the appl ...
... a. To learn preparations reactions and properties of alkane, alkene and alkynes. b. To learn estimation of nitrogen and molecular weight determination of organic acids and bases c. To learn basics of selected organic reactions and mechanisms d. To understand the atomic structure e. To learn the appl ...
Acrobat () verson
... examination, but rather, it consists of a collection of short problems that provide the student an opportunity to display her or his problem-solving abilities. On such questions, major partial credit is awarded for the development of an approach that will lead to a successful answer. So, make sure t ...
... examination, but rather, it consists of a collection of short problems that provide the student an opportunity to display her or his problem-solving abilities. On such questions, major partial credit is awarded for the development of an approach that will lead to a successful answer. So, make sure t ...