AP Chemistry
... 1. one directional reaction: reactants products 2. equilibrium reaction: "reactants" "products" conservation of atoms (mass)—Dalton's Theory ...
... 1. one directional reaction: reactants products 2. equilibrium reaction: "reactants" "products" conservation of atoms (mass)—Dalton's Theory ...
Chapter 15. Chemical Equilibrium
... • That is, the system shifts to remove gases and decrease pressure. • An increase in pressure favors the direction that has fewer moles of gas. Consider the following system: N2O4(g) 2NO2(g) • An increase in pressure (by decreasing the volume) favors the formation of colorless N2O4. • The instant ...
... • That is, the system shifts to remove gases and decrease pressure. • An increase in pressure favors the direction that has fewer moles of gas. Consider the following system: N2O4(g) 2NO2(g) • An increase in pressure (by decreasing the volume) favors the formation of colorless N2O4. • The instant ...
Chemistry
... Chemistry 361, Chemistry 374, Chemistry 482 OR 483 and two Chemistry electives, at least one of which is at the 4th year level. Additional course requirements for the Chemistry major include the following courses from other disciplines: Biology 131-132, Mathematics 191, Mathematics 192, Mathematics ...
... Chemistry 361, Chemistry 374, Chemistry 482 OR 483 and two Chemistry electives, at least one of which is at the 4th year level. Additional course requirements for the Chemistry major include the following courses from other disciplines: Biology 131-132, Mathematics 191, Mathematics 192, Mathematics ...
Chem 2A Final Review
... ans. Ba(OH)2 (aq) , BaSO4 (s) , BaS (s) , Ba(NO3)2 (aq) 40. Ans. (0.220 g NaCl/100 mL solution) 65.0 mL solution = 0.143 g NaCl 41. Ans. 12.1 g NaCl /(12.1 g NaCl + 150.1 g water) 100 = 7.57 % 42. M = mol/L, mol = M L, 50.0 mL 1L/1000mL 12.0 M = 0.600 mol HNO3, mol = g/mw, g = mol mw = 0 ...
... ans. Ba(OH)2 (aq) , BaSO4 (s) , BaS (s) , Ba(NO3)2 (aq) 40. Ans. (0.220 g NaCl/100 mL solution) 65.0 mL solution = 0.143 g NaCl 41. Ans. 12.1 g NaCl /(12.1 g NaCl + 150.1 g water) 100 = 7.57 % 42. M = mol/L, mol = M L, 50.0 mL 1L/1000mL 12.0 M = 0.600 mol HNO3, mol = g/mw, g = mol mw = 0 ...
Stoichiometry notes 1
... Can be used as a conversion factor between moles and Number of representative particles. Reminder: The representative particles for 1. an element is an atom 2. for a molecular compound is a molecule 3. for an ionic compound is a formula unit ...
... Can be used as a conversion factor between moles and Number of representative particles. Reminder: The representative particles for 1. an element is an atom 2. for a molecular compound is a molecule 3. for an ionic compound is a formula unit ...
Chemistry
... 54. Which of the following reagents can convert acetic acid into ethanol ? (A) Sn + HCl (B) H2 + Pt (C) LiAlH4 + ether (D) H2 + Ni 55. An organic compound ‘X’ is oxidized by using acidified K2Cr2O7. The product obtained reacts with phenyl hydrazine but does not give silver mirror test. The possible ...
... 54. Which of the following reagents can convert acetic acid into ethanol ? (A) Sn + HCl (B) H2 + Pt (C) LiAlH4 + ether (D) H2 + Ni 55. An organic compound ‘X’ is oxidized by using acidified K2Cr2O7. The product obtained reacts with phenyl hydrazine but does not give silver mirror test. The possible ...
File
... 5.2 Oxidation Numbers Oxidation number the apparent charge an atom would have if it gained or lost its bonding electrons Consider the example of sulfur dioxide, SO2. In sulfur dioxide, oxygen is more electronegative than sulfur. Since oxygen gains two electrons to form the oxide ion, O-2, in ionic c ...
... 5.2 Oxidation Numbers Oxidation number the apparent charge an atom would have if it gained or lost its bonding electrons Consider the example of sulfur dioxide, SO2. In sulfur dioxide, oxygen is more electronegative than sulfur. Since oxygen gains two electrons to form the oxide ion, O-2, in ionic c ...
17.2 The Avogadro Number
... unbalance another. By the end of the process, however, all elements will be balanced. We now have the choice of balancing either the iodine or the sodium. Let's balance the iodine. (It doesn’t matter which element we choose.) There are two atoms of iodine on the reactant side of the equation and onl ...
... unbalance another. By the end of the process, however, all elements will be balanced. We now have the choice of balancing either the iodine or the sodium. Let's balance the iodine. (It doesn’t matter which element we choose.) There are two atoms of iodine on the reactant side of the equation and onl ...
Lipid Hydroperoxide Activation of N-Hydroxy-N
... previously (7). The solvent system used was dichlorometh ane/acetone (85/5, v/v). In the hematin/N-OH-AAF/LAHP system, incubations were the same as those described for the optical and ESR studies, except that larger volumes were used. Extraction and TLC of this system were con ducted as with the per ...
... previously (7). The solvent system used was dichlorometh ane/acetone (85/5, v/v). In the hematin/N-OH-AAF/LAHP system, incubations were the same as those described for the optical and ESR studies, except that larger volumes were used. Extraction and TLC of this system were con ducted as with the per ...
File
... Spring 2012, Lecture test II in chemistry 2. Name___________________________ Answer questions 1 to 19 on these pages. ______1. Which of the following will increase the Ksp of PbCl2 ? A) Addition of HCl to the solution B) Addition of Pb(NO3)2 to the solution C) An increase in temperature D) All of th ...
... Spring 2012, Lecture test II in chemistry 2. Name___________________________ Answer questions 1 to 19 on these pages. ______1. Which of the following will increase the Ksp of PbCl2 ? A) Addition of HCl to the solution B) Addition of Pb(NO3)2 to the solution C) An increase in temperature D) All of th ...
Chapter 3 Stoichiometry: Calculations with Chemical
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this – C is determined from the mass of CO2 produced – H is determined from the mass of H2O produced – O is determined by difference after the C and H have been ...
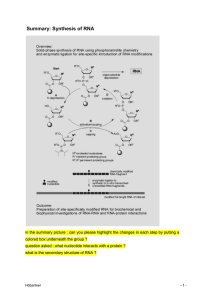
Synthesis of RNA - Stamm revision
... families of phosphoramidite building blocks for RNA solid-phase synthesis (Figure 1A) belong to two classes of orthogonal protecting group strategies, the 5’-O-DMT-2’-O-silyl (1 and 2) and the 5’-O-silyl-2’-O-ACE (3) strategy. Can you explain here DMT and ACE The tert-butyldimethylsilyl (TBDMS) grou ...
... families of phosphoramidite building blocks for RNA solid-phase synthesis (Figure 1A) belong to two classes of orthogonal protecting group strategies, the 5’-O-DMT-2’-O-silyl (1 and 2) and the 5’-O-silyl-2’-O-ACE (3) strategy. Can you explain here DMT and ACE The tert-butyldimethylsilyl (TBDMS) grou ...
Reactants Products
... mole of I2 will also be used and 2 moles of HI made. – Therefore, the rate of change will be different. ...
... mole of I2 will also be used and 2 moles of HI made. – Therefore, the rate of change will be different. ...
Chapter 5 Atomic Structure
... 2. Solutions of problems involving the relationships between the number of particles, the amount of substance in moles and the mass in grams. 3. Inter conversion of the percentage composition by mass and the empirical formula 4. Determination of the molecular formula of a compound from its empirical ...
... 2. Solutions of problems involving the relationships between the number of particles, the amount of substance in moles and the mass in grams. 3. Inter conversion of the percentage composition by mass and the empirical formula 4. Determination of the molecular formula of a compound from its empirical ...
K eq
... 1. Each student wads up two paper wads. 2. You must start and stop as the timekeeper says. 3. Throw only one paper wad at a time. 4. If a paper wad lands next to you, you must throw it back. ...
... 1. Each student wads up two paper wads. 2. You must start and stop as the timekeeper says. 3. Throw only one paper wad at a time. 4. If a paper wad lands next to you, you must throw it back. ...