CHAPTER VI Fischer-Tropsch Refineries
... in the tubes to regulate the temperature. The catalyst bed was typically operated 5-8°C higher than the temperature in the tubes and in practise the reactor temperature could be controlled to within 1°C in the range 170-200°C by regulating the water pressure, using a boiler principle. c The synthesi ...
... in the tubes to regulate the temperature. The catalyst bed was typically operated 5-8°C higher than the temperature in the tubes and in practise the reactor temperature could be controlled to within 1°C in the range 170-200°C by regulating the water pressure, using a boiler principle. c The synthesi ...
Formatting Blackline Masters
... Note: A represents the central atom in the molecule. B represents atoms bonded to the central atom. B can be identical atoms or different atoms. Directions: 1. Find the other students who have the same color balloons as you. Have someone inflate a balloon as much as possible without popping it. Infl ...
... Note: A represents the central atom in the molecule. B represents atoms bonded to the central atom. B can be identical atoms or different atoms. Directions: 1. Find the other students who have the same color balloons as you. Have someone inflate a balloon as much as possible without popping it. Infl ...
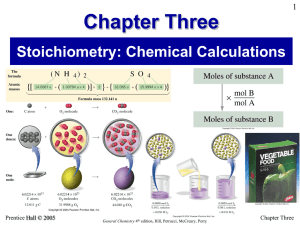
Chapter Three
... Characteristics of a Chemical Equation • 1. The equation must represent known facts. All reactants and products MUST be identified. • 2. The equation must contain the correct formulas for the reactants and products. • 3. The law of conservation of mass must be satisfied. • NOTE = we are ONLY allowed ...
... Characteristics of a Chemical Equation • 1. The equation must represent known facts. All reactants and products MUST be identified. • 2. The equation must contain the correct formulas for the reactants and products. • 3. The law of conservation of mass must be satisfied. • NOTE = we are ONLY allowed ...
Mass Relationships in Chemical Reactions
... (vitamin C: cures/prevents scurvy). It is composed of 40.92% C, 4.58% H, and 54.50% O by mass. To determine the empirical formula, we will first assume a 100 g sample of Ascorbic Acid ...
... (vitamin C: cures/prevents scurvy). It is composed of 40.92% C, 4.58% H, and 54.50% O by mass. To determine the empirical formula, we will first assume a 100 g sample of Ascorbic Acid ...
AP® Chemistry
... The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded in 1900, the association is composed of more than 5,000 schools, colleges, universities, and other educational organizations. Each year, the College Board ser ...
... The College Board is a not-for-profit membership association whose mission is to connect students to college success and opportunity. Founded in 1900, the association is composed of more than 5,000 schools, colleges, universities, and other educational organizations. Each year, the College Board ser ...
03_Worked_Examples
... (a) The left box, which represents reactants, contains two kinds of molecules, those composed of two oxygen atoms (O2) and those composed of one nitrogen atom and one oxygen atom (NO). The right box, which represents products, contains only one kind of molecule, which is composed of one nitrogen ato ...
... (a) The left box, which represents reactants, contains two kinds of molecules, those composed of two oxygen atoms (O2) and those composed of one nitrogen atom and one oxygen atom (NO). The right box, which represents products, contains only one kind of molecule, which is composed of one nitrogen ato ...
U6B _13-14
... KI(aq) + AgNO3(aq) AgI(s) + KNO3(aq) Complete Ionic equation: K+1 + I-1 + Ag+1+ NO3-1 AgI + K+1 + NO3-1 Spectator ions: ions that do not participate in a reaction; they are identical on both sides of the equation & are crossed out! ...
... KI(aq) + AgNO3(aq) AgI(s) + KNO3(aq) Complete Ionic equation: K+1 + I-1 + Ag+1+ NO3-1 AgI + K+1 + NO3-1 Spectator ions: ions that do not participate in a reaction; they are identical on both sides of the equation & are crossed out! ...
Ch 17 Equilibrium
... to lower the pressure, because there are left fewer moles of gas on that side of the equation. ...
... to lower the pressure, because there are left fewer moles of gas on that side of the equation. ...
Kinetics Workbook - School District 67
... This workbook will allow you to demonstrate your understanding of all aspects of the kinetics unit. The minimum expectation is that you do all of these questions by the due dates given by your teacher. Do the questions. Use your notes from class to assist you. Then after you have finished go to the ...
... This workbook will allow you to demonstrate your understanding of all aspects of the kinetics unit. The minimum expectation is that you do all of these questions by the due dates given by your teacher. Do the questions. Use your notes from class to assist you. Then after you have finished go to the ...
Physical chemistry and transition elements 5.1 Rates, equilibrium
... ∆S is the entropy change for the reaction (measured in J mol−1 K−1) [1] T is temperature in K Both units and definition needed for each mark. (i) Endothermic reactions have a +∆H value [1]. For an endothermic reaction to be feasible ∆G must be negative [1]. This means that if ∆S is positive and T is ...
... ∆S is the entropy change for the reaction (measured in J mol−1 K−1) [1] T is temperature in K Both units and definition needed for each mark. (i) Endothermic reactions have a +∆H value [1]. For an endothermic reaction to be feasible ∆G must be negative [1]. This means that if ∆S is positive and T is ...
AP Chemistry - Notes
... b. conservation of atoms (mass) - atoms are neither created nor destroyed in chemical reactions, they are recombined to form different substances - mass is neither created nor destroyed chemical reactions (as opposed to nuclear reactions) - chemical reactions must therefore be balanced - have same k ...
... b. conservation of atoms (mass) - atoms are neither created nor destroyed in chemical reactions, they are recombined to form different substances - mass is neither created nor destroyed chemical reactions (as opposed to nuclear reactions) - chemical reactions must therefore be balanced - have same k ...
California Standards Practice - Student Edition
... table to its atomic number and atomic mass. b. Students know how to use the periodic table to identify metals, semimetals, nonmetals, and halogens. c. Students know how to use the periodic table to identify alkali metals, alkaline earth metals and transition metals, trends in ionization energy, elec ...
... table to its atomic number and atomic mass. b. Students know how to use the periodic table to identify metals, semimetals, nonmetals, and halogens. c. Students know how to use the periodic table to identify alkali metals, alkaline earth metals and transition metals, trends in ionization energy, elec ...
IB Chemistry HL Topic5 Questions 1. Which combination of ionic
... Which equation represents a change with a negative value for S? A. ...
... Which equation represents a change with a negative value for S? A. ...
Chapter 5
... the carbonylative cycloaddition of allyl halides and alkynes with Ni(CO) 4 by Chiusoli2 and the Pauson-Khand reaction3 (M n (CO) n =Co2 (CO) 6 ) in the early 1970s, it has been found that many other transition metal complexes can undergo cyclocondensations: Ti,4 Zr, 5 Mo, 6 W, 7 Fe, 8 Ru, 9 Ni, 10 a ...
... the carbonylative cycloaddition of allyl halides and alkynes with Ni(CO) 4 by Chiusoli2 and the Pauson-Khand reaction3 (M n (CO) n =Co2 (CO) 6 ) in the early 1970s, it has been found that many other transition metal complexes can undergo cyclocondensations: Ti,4 Zr, 5 Mo, 6 W, 7 Fe, 8 Ru, 9 Ni, 10 a ...
expected output
... To produce industrial chemists who can work as quality controllers (from raw material and intermediate product to final product) in every sector of the industry. To produce a personal capable to work in research laboratories, standardization laboratories, clinical laboratories, college and universit ...
... To produce industrial chemists who can work as quality controllers (from raw material and intermediate product to final product) in every sector of the industry. To produce a personal capable to work in research laboratories, standardization laboratories, clinical laboratories, college and universit ...
Organic Reactions in Organised Media
... that are formed, the rate of such reactions is low unless special measures are taken. Typical reactions facing such a problem are nucleophilic substitution reactions between a lipophilic organic compound and an inorganic ion, hydrolysis of organic compounds by caustic, many electrophilic substitutio ...
... that are formed, the rate of such reactions is low unless special measures are taken. Typical reactions facing such a problem are nucleophilic substitution reactions between a lipophilic organic compound and an inorganic ion, hydrolysis of organic compounds by caustic, many electrophilic substitutio ...
expected output
... To produce industrial chemists who can work as quality controllers (from raw material and intermediate product to final product) in every sector of the industry. To produce a personal capable to work in research laboratories, standardization laboratories, clinical laboratories, college and universit ...
... To produce industrial chemists who can work as quality controllers (from raw material and intermediate product to final product) in every sector of the industry. To produce a personal capable to work in research laboratories, standardization laboratories, clinical laboratories, college and universit ...
9/10/10 1 Chemistry 121: Atomic and Molecular Chemistry
... substances. Recall previous example with NH3. A chemical equation uses chemical symbols to show what happens in a chemical reaction. Writing Chemical Equations: When hydrogen gas (H2) burns in air (which contains oxygen, O2) to form water (H2O). H2 + O2 → H2O This symbolic expression can be read: "M ...
... substances. Recall previous example with NH3. A chemical equation uses chemical symbols to show what happens in a chemical reaction. Writing Chemical Equations: When hydrogen gas (H2) burns in air (which contains oxygen, O2) to form water (H2O). H2 + O2 → H2O This symbolic expression can be read: "M ...