Chapter 16 Controlling the yield of reactions
... 2HI(g) H2(g) + I2(g) is 48.8 at 455°C. An equilibrium mixture in a 2.0 L vessel at this temperature contains 0.220 mol of H2 and 0.110 mol of I2. a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0. ...
... 2HI(g) H2(g) + I2(g) is 48.8 at 455°C. An equilibrium mixture in a 2.0 L vessel at this temperature contains 0.220 mol of H2 and 0.110 mol of I2. a Calculate the concentration of HI in this mixture. b Another mixture was prepared by placing 4.0 mol of HI in a 2.0 L vessel at 330°C. At equilibrium 0. ...
PDF File
... the observed K1/2 values equal the dissociation constants Kd (see also ref 37): The same K1/2 values were observed in concentration dependences in which the maximal rate constant for reaction varied by more than 10-fold, which was accomplished by a 2′-H substitution at position -1 and by varying the ...
... the observed K1/2 values equal the dissociation constants Kd (see also ref 37): The same K1/2 values were observed in concentration dependences in which the maximal rate constant for reaction varied by more than 10-fold, which was accomplished by a 2′-H substitution at position -1 and by varying the ...
CHEMISTRY CHM-050 Introduction to Chemistry I NCC Cr: 3 D Lec
... Lec Y Lab: Y Prerequisite: MAT-063, Elementary Algebra, or equivalent. A onesemester college chemistry course which surveys important concepts and topics of chemistry. Among these are the metric system of measurement, atomic theory of matter, energy levels and atomic structure, the periodic table, i ...
... Lec Y Lab: Y Prerequisite: MAT-063, Elementary Algebra, or equivalent. A onesemester college chemistry course which surveys important concepts and topics of chemistry. Among these are the metric system of measurement, atomic theory of matter, energy levels and atomic structure, the periodic table, i ...
B.Sc. (Hons.) Chemistry
... 6. Organometallics, Bioinorganic chemistry, Polynuclear hydrocarbons and UV, IR Spectroscopy 7. Molecules of life (4) + Lab (4). Note: Universities may include more options or delete some from this list Important: 1. Each University/Institute should provide a brief write-up about each paper outlinin ...
... 6. Organometallics, Bioinorganic chemistry, Polynuclear hydrocarbons and UV, IR Spectroscopy 7. Molecules of life (4) + Lab (4). Note: Universities may include more options or delete some from this list Important: 1. Each University/Institute should provide a brief write-up about each paper outlinin ...
Chapter 16: Reaction Rates
... Collision orientation and the activated complex Why do most collisions fail to produce products? What other factors must be considered? Figure 16.4a and b show one possible answer to this question. These illustrations indicate that in order for a collision to lead to a reaction, the carbon atom in a ...
... Collision orientation and the activated complex Why do most collisions fail to produce products? What other factors must be considered? Figure 16.4a and b show one possible answer to this question. These illustrations indicate that in order for a collision to lead to a reaction, the carbon atom in a ...
Proposed syllabus and Scheme of Examination B.Sc. (Program) with
... Atomic Structure: Review of: Bohr’s theory and its limitations, dual behaviour of matter and radiation, de-Broglie’s relation, Heisenberg Uncertainty principle. Hydrogen atom spectra. Need of a new approach to Atomic structure. What is Quantum mechanics? Time independent Schrodinger equation and mea ...
... Atomic Structure: Review of: Bohr’s theory and its limitations, dual behaviour of matter and radiation, de-Broglie’s relation, Heisenberg Uncertainty principle. Hydrogen atom spectra. Need of a new approach to Atomic structure. What is Quantum mechanics? Time independent Schrodinger equation and mea ...
Document
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...
Chapter
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...
Chapter 14 (Kinetics) – Slides and Practice
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...
... Measuring Reaction Rate • To measure the reaction rate you need to be able to measure the concentration of at least one component in the mixture at many points in time • There are two ways of approaching this problem 1. for reactions that are complete in less than 1 hour, it is best to use continuo ...
Higher Chemistry Resources Guide - Glow Blogs
... A potential energy diagram can be used to show the energy pathway for a reaction. The enthalpy change is the energy difference between products and reactants. It can be calculated from a potential energy diagram. The enthalpy change has a negative value for exothermic reactions and a positive value ...
... A potential energy diagram can be used to show the energy pathway for a reaction. The enthalpy change is the energy difference between products and reactants. It can be calculated from a potential energy diagram. The enthalpy change has a negative value for exothermic reactions and a positive value ...
CHEMICAL EQUILIBRIUM (Part II)II)
... Constants Knowing the equilibrium constant (Kc or ) and/or the initial concentrations of KP) and/or the initial concentrations of reactants and products for a given reaction allows you to predict several features of that reaction, such as: o whether the reaction tends to occur or not. or not ...
... Constants Knowing the equilibrium constant (Kc or ) and/or the initial concentrations of KP) and/or the initial concentrations of reactants and products for a given reaction allows you to predict several features of that reaction, such as: o whether the reaction tends to occur or not. or not ...
SCH4U Exam Review
... Is the system in the state of equilibrium? If not which direction will the reaction have to proceed to reach equilibrium? ANS: k=8.242, shifts left ...
... Is the system in the state of equilibrium? If not which direction will the reaction have to proceed to reach equilibrium? ANS: k=8.242, shifts left ...
Syllabus and Regulations for 2-year, 4
... Semester may be composed of the following components: experimental / theoretical / review. The project report has to be submitted by the respective candidates (in triplicate) in the form of dissertations. The dissertation part (30 marks) and the seminar part (20 marks) shall be evaluated by a Board ...
... Semester may be composed of the following components: experimental / theoretical / review. The project report has to be submitted by the respective candidates (in triplicate) in the form of dissertations. The dissertation part (30 marks) and the seminar part (20 marks) shall be evaluated by a Board ...
Ch3
... Solve the following conversions How many atoms of silver are in 3.50 moles of silver? Determine the number of moles of carbon disulfide in 34.75 grams of CS2. Determine the number of sulfur atoms in 34.75 grams of CS2. Copyright McGraw-Hill 2009 ...
... Solve the following conversions How many atoms of silver are in 3.50 moles of silver? Determine the number of moles of carbon disulfide in 34.75 grams of CS2. Determine the number of sulfur atoms in 34.75 grams of CS2. Copyright McGraw-Hill 2009 ...
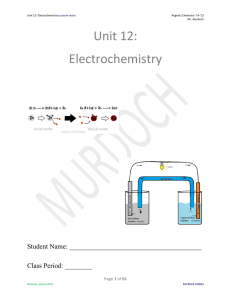
Unit 12: Electrochemistry
... Objective: Why do we study electricity in chemistry? Electricity: Why study electricity in chemistry? Isn’t that a physics topic? Well, yes it is, as I have taught Regents physics as well. But to understand what you can DO with electricity in physics, you need to understand how electricity is create ...
... Objective: Why do we study electricity in chemistry? Electricity: Why study electricity in chemistry? Isn’t that a physics topic? Well, yes it is, as I have taught Regents physics as well. But to understand what you can DO with electricity in physics, you need to understand how electricity is create ...
Higher Chemistry Resources Guide - Glow Blogs
... additional fourth column has been included which contains hyperlinks to useful resources. Please note: Practitioners are not required to use the resources listed – they are only included as helpful suggestions. Practitioners should also refer to the SQA website for the most up to date Course and Uni ...
... additional fourth column has been included which contains hyperlinks to useful resources. Please note: Practitioners are not required to use the resources listed – they are only included as helpful suggestions. Practitioners should also refer to the SQA website for the most up to date Course and Uni ...
Reactions of Plutonium Dioxide with Water and Oxygen
... (approximately 0.1 g) were accurately weighed prior to placement in the Pt sample container. The oxide was outgassed to constant mass in vacuum at 400eC and was then exposed to water pressure at 15 tom as the temperature was increased stepwise from 25°C to 50, 100, 150, and 250°C over a period in ex ...
... (approximately 0.1 g) were accurately weighed prior to placement in the Pt sample container. The oxide was outgassed to constant mass in vacuum at 400eC and was then exposed to water pressure at 15 tom as the temperature was increased stepwise from 25°C to 50, 100, 150, and 250°C over a period in ex ...
Honors Chemistry
... elements can combine in simple whole number ratios to form compounds. Such is the case in the reaction of lead (II) nitrate with sodium iodide. This laboratory investigation will demonstrate this fact and help you understand molar relationships in reactions and their importance in the chemical produ ...
... elements can combine in simple whole number ratios to form compounds. Such is the case in the reaction of lead (II) nitrate with sodium iodide. This laboratory investigation will demonstrate this fact and help you understand molar relationships in reactions and their importance in the chemical produ ...
Stoichiometry of Ozonation of Environmentally
... organics in a well-defined stoichiometric ratio, with one carbon-carbon double bond consumed per ozone molecule reacted. However, this work demonstrates that a single ozone molecule can effectively destroy several double bonds in dilute solutions of monoterpenes and unsaturated fatty acids via a cha ...
... organics in a well-defined stoichiometric ratio, with one carbon-carbon double bond consumed per ozone molecule reacted. However, this work demonstrates that a single ozone molecule can effectively destroy several double bonds in dilute solutions of monoterpenes and unsaturated fatty acids via a cha ...
Stoichiometry: Calculations with Chemical Formulas and Equations
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this. – C is determined from the mass of CO2 produced. – H is determined from the mass of H2O produced. – O is determined by difference after the C and H have been ...
... • Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this. – C is determined from the mass of CO2 produced. – H is determined from the mass of H2O produced. – O is determined by difference after the C and H have been ...