A2 Chemistry key word list
... infrared radiation by atmospheric gases warms the lower atmosphere and the planet’s surface. ...
... infrared radiation by atmospheric gases warms the lower atmosphere and the planet’s surface. ...
Building Atoms Unit Interactive Science Notebook III
... 4. For which isotopes have scientists not been able to determine the atomic mass? Can you think of a reason for this? 5. According to the table, how are isotopes named? 6. What is the atomic number for all the isotopes of carbon? __________ 7.What is the atomic number for all the isotopes of oxygen? ...
... 4. For which isotopes have scientists not been able to determine the atomic mass? Can you think of a reason for this? 5. According to the table, how are isotopes named? 6. What is the atomic number for all the isotopes of carbon? __________ 7.What is the atomic number for all the isotopes of oxygen? ...
Ch2hon ppt part 3
... Write chemical formulas for the following molecular compounds: (a) carbon disulfide (b) disilicon hexabromide ...
... Write chemical formulas for the following molecular compounds: (a) carbon disulfide (b) disilicon hexabromide ...
How to balance chemical equations File
... When you write an equation for a chemical reaction, the two sides of the equation should balance — you need the same number of each kind of element on both sides. If you carry out a chemical reaction and carefully sum up the masses of all the reactants, and then compare the sum to the sum of the mas ...
... When you write an equation for a chemical reaction, the two sides of the equation should balance — you need the same number of each kind of element on both sides. If you carry out a chemical reaction and carefully sum up the masses of all the reactants, and then compare the sum to the sum of the mas ...
atom - Zanichelli online per la scuola
... In order to balance a chemical equation it is a good practice to follow these procedures: 1.balance the atoms of metals and non metals first; 2.even when they are present in compounds, oxygen and hydrogen should be balanced after the others, because they often appear in many formulae; 3.balance wate ...
... In order to balance a chemical equation it is a good practice to follow these procedures: 1.balance the atoms of metals and non metals first; 2.even when they are present in compounds, oxygen and hydrogen should be balanced after the others, because they often appear in many formulae; 3.balance wate ...
Unit 2 - Solon City Schools
... 3)______________________ Mass is neither created nor destroyed. 4)______________________ Value represented by # of protons + # of neutrons. 5)______________________ He founded the “mathematical” model of the atom. The electrons are located in the electron cloud. 6)______________________ The one elem ...
... 3)______________________ Mass is neither created nor destroyed. 4)______________________ Value represented by # of protons + # of neutrons. 5)______________________ He founded the “mathematical” model of the atom. The electrons are located in the electron cloud. 6)______________________ The one elem ...
Atomic Structure Note Packet
... _____________ = SI unit for amount of substance A mole simply represents a______________________, much in the same way that a dozen represents a set of twelve. Ex: Just as you can have a dozen cans of soda or a dozen donuts, you can have a mole of stars or a mole of water molecules. So a dozen eggs ...
... _____________ = SI unit for amount of substance A mole simply represents a______________________, much in the same way that a dozen represents a set of twelve. Ex: Just as you can have a dozen cans of soda or a dozen donuts, you can have a mole of stars or a mole of water molecules. So a dozen eggs ...
Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for anti-viral therapy.
... Flux profiling was conducted 48 h after HCMV infection, when large changes in metabolite concentrations first appear18. A battery of five assays was used: (i) direct measurement of key cellular metabolic influxes (glucose and glutamine) and effluxes (pyruvate, lactate, alanine and glutamate), (ii) k ...
... Flux profiling was conducted 48 h after HCMV infection, when large changes in metabolite concentrations first appear18. A battery of five assays was used: (i) direct measurement of key cellular metabolic influxes (glucose and glutamine) and effluxes (pyruvate, lactate, alanine and glutamate), (ii) k ...
(hydroxypyridin-3-yl-methyl)phosphonic acid
... to labile protons existing in the structure of the ligand. This ligand contains two dissociable protons within the measurable pH range (2.0 – 12.0). On the other hand, one more deprotonation has been postulated at pH below 2.0. Based on these data we propose four forms that result from consecutive d ...
... to labile protons existing in the structure of the ligand. This ligand contains two dissociable protons within the measurable pH range (2.0 – 12.0). On the other hand, one more deprotonation has been postulated at pH below 2.0. Based on these data we propose four forms that result from consecutive d ...
10 IB Chemistry Assessment Statements 2009 Revised
... Int, aim 8: Today, we may be starting to experience the consequences of using fossil fuels as our main source of energy. There is a vast range of products that can be derived from fossil fuels as a result of carbon’s rich chemistry. This raises the question “are they too valuable to burn?”. ...
... Int, aim 8: Today, we may be starting to experience the consequences of using fossil fuels as our main source of energy. There is a vast range of products that can be derived from fossil fuels as a result of carbon’s rich chemistry. This raises the question “are they too valuable to burn?”. ...
Atoms and Elements Atoms and Elements
... protons & neutrons in nucleus # electrons = # protons electrons in space around nucleus Extremely small! One teaspoon of water has 3 times as many atoms as the Atlantic Ocean has teaspoons of water. MAR ...
... protons & neutrons in nucleus # electrons = # protons electrons in space around nucleus Extremely small! One teaspoon of water has 3 times as many atoms as the Atlantic Ocean has teaspoons of water. MAR ...
Calculations with Chemical Formulas and Equations
... sense since propane is the material that you must purchase in order to cook your food. b. Since the chemical reaction only requires propane and oxygen, if the grill will not light with ample propane present, then the limiting reactant must be oxygen. c. Once again, here is a case where you have adeq ...
... sense since propane is the material that you must purchase in order to cook your food. b. Since the chemical reaction only requires propane and oxygen, if the grill will not light with ample propane present, then the limiting reactant must be oxygen. c. Once again, here is a case where you have adeq ...
Unit 1. Materials: Formulating Matter A. How do chemists describe
... The U.S. Mint is promoting the use of dollar coins instead of dollar bills. In what ways might coins be superior to bills? How do the properties of each form of currency depend upon their composition? In this unit, you will consider the benefits and drawbacks of using a dollar coin. You will make re ...
... The U.S. Mint is promoting the use of dollar coins instead of dollar bills. In what ways might coins be superior to bills? How do the properties of each form of currency depend upon their composition? In this unit, you will consider the benefits and drawbacks of using a dollar coin. You will make re ...
4.1 Section Assessment
... From his experiments, Rutherford concluded that an atom is made of a positively-charged nucleus surrounded by a region of empty space in which electrons orbit that nucleus. Rutherford believed that an atom’s nucleus was very tiny compared to the atom as a whole, and that, in spite of this, the nucle ...
... From his experiments, Rutherford concluded that an atom is made of a positively-charged nucleus surrounded by a region of empty space in which electrons orbit that nucleus. Rutherford believed that an atom’s nucleus was very tiny compared to the atom as a whole, and that, in spite of this, the nucle ...
PP Chapter 2
... Have you ever sat around a campfire or watched flames flicker in a fireplace? The burning of wood is a chemical reaction—a process that changes one set of chemicals into another set of chemicals. A chemical reaction always involves changes in chemical bonds that join atoms in compounds. The elements ...
... Have you ever sat around a campfire or watched flames flicker in a fireplace? The burning of wood is a chemical reaction—a process that changes one set of chemicals into another set of chemicals. A chemical reaction always involves changes in chemical bonds that join atoms in compounds. The elements ...
Chapter 3
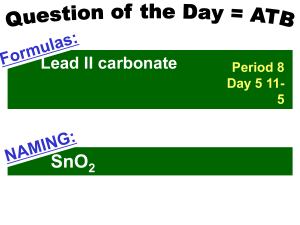
... number 1 subscript is left out Formula contains more than one atom of the same element – the number is indicated as a subscript written to the right of the symbol of that atom When a formula contains more than one group of atoms occur as a unit – a parentheses is place around the group with the ...
... number 1 subscript is left out Formula contains more than one atom of the same element – the number is indicated as a subscript written to the right of the symbol of that atom When a formula contains more than one group of atoms occur as a unit – a parentheses is place around the group with the ...
File
... • To balance an equation means to change the numbers of each molecule involved, so that the same number of atoms of each element appear on the reactants side and on the products side. • Chemical equations balance on an atomic level, not molecular. • You cannot change the formula of a substance, i.e. ...
... • To balance an equation means to change the numbers of each molecule involved, so that the same number of atoms of each element appear on the reactants side and on the products side. • Chemical equations balance on an atomic level, not molecular. • You cannot change the formula of a substance, i.e. ...
document
... Solid: A substance that has a definite shape and volume. Liquid: A substance that has a definite volume but that changes shape to fill the container. Gas: A substance that has neither a definite volume nor a definite shape. Many substances, such as water, can exist in all three states depending on t ...
... Solid: A substance that has a definite shape and volume. Liquid: A substance that has a definite volume but that changes shape to fill the container. Gas: A substance that has neither a definite volume nor a definite shape. Many substances, such as water, can exist in all three states depending on t ...
Pentose Phosphate Pathway - Lectures For UG-5
... • The oxidative decarboxylation of the product, 6phosphogluconate is catalyzed by 6phosphogluconate dehydrogenase. • This irreversible reaction produces a pentose sugar-phosphate (ribufose 5-phosphate), CO2 (from carbon 1 of glucose), and a second molecule of NADPH (Figure 13.2). ...
... • The oxidative decarboxylation of the product, 6phosphogluconate is catalyzed by 6phosphogluconate dehydrogenase. • This irreversible reaction produces a pentose sugar-phosphate (ribufose 5-phosphate), CO2 (from carbon 1 of glucose), and a second molecule of NADPH (Figure 13.2). ...
Ch6-Energy in Chemical Reactions-Chemical Reactions
... Chemical Reactions: Enthalpy and Hesse’s Law Calculations Why? In chemistry, the mole is the standard measurement of amount. When substances react according to chemical equations, they do so in simple ratios of moles. However, balances give readings in grams. Balances DO NOT give readings in moles. ...
... Chemical Reactions: Enthalpy and Hesse’s Law Calculations Why? In chemistry, the mole is the standard measurement of amount. When substances react according to chemical equations, they do so in simple ratios of moles. However, balances give readings in grams. Balances DO NOT give readings in moles. ...
Chemical Formulas and Composition Stoichiometry
... • An element is composed of extremely small, indivisible particles called _____. • All atoms of a given element have ______ properties that are ______. • Atoms cannot be _____, ______, or ______ into atoms of another element. • ______ are formed when atoms of different elements combine with one anot ...
... • An element is composed of extremely small, indivisible particles called _____. • All atoms of a given element have ______ properties that are ______. • Atoms cannot be _____, ______, or ______ into atoms of another element. • ______ are formed when atoms of different elements combine with one anot ...
Example
... For the reaction, determine the theoretical yield if one starts with 1.20 g of antimony and 2.40 g of iodine. 2 Sb(s) + 3 I2(s) 2 SbI3(s) ...
... For the reaction, determine the theoretical yield if one starts with 1.20 g of antimony and 2.40 g of iodine. 2 Sb(s) + 3 I2(s) 2 SbI3(s) ...
ChemistryofLifeOLDve..
... Organic molecules are any molecules that contain atoms from three elements: carbon, hydrogen, and oxygen. For example, glucose is organic, since its molecular formula is C6H12O6 Carbon dioxide (CO2) is inorganic since it does not contain hydrogen. Covalent bonds link carbon atoms together in long ch ...
... Organic molecules are any molecules that contain atoms from three elements: carbon, hydrogen, and oxygen. For example, glucose is organic, since its molecular formula is C6H12O6 Carbon dioxide (CO2) is inorganic since it does not contain hydrogen. Covalent bonds link carbon atoms together in long ch ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.