* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 3
X-ray fluorescence wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Bose–Einstein condensate wikipedia , lookup
Cluster chemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Electron configuration wikipedia , lookup
Isotopic labeling wikipedia , lookup
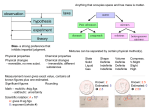
Chapter 3 – Classification of Matter Elements – Distribution, Names, Symbols Elements - Metals, Nonmetals, Metalloids Diatomic Molecules Chemical Formulas Mixtures Elements Element: A substance that cannot be decomposed into simpler substances by chemical means. Make up our chemical alphabet. Over 100 known elements Elements Element: Our building block of all substances. Numbered in order of increasing complexity •Elements through 92 are known to occur in nature. [with 4 exception Technetium (43), Promethium (61), Astatine (85), Francium (87)] •Above 92 only Plutonium (94) occur in nature. •Above 92 all elements must be synthesized in the laboratories in small quantities Elements Most substances can be decomposed into two or more simpler substance. Water = Hydrogen and Oxygen Sugar = Carbon, Hydrogen, and Oxygen Salt = Sodium and Chloride Elements The smallest particle of an element that can exist is an atom, which is also the smallest unit of an element that can enter into a chemical reaction. Atoms are made up of subatomic particles that will be discussed later in the semester. Distribution of Elements Elements are distributed unequally in nature Ten elements make up 99% of the mass of the Earths Crust, seawater, and atmosphere Oxygen is about 50% of this mass Two elements are liquids at room temperature Bromine and Mercury Eleven elements are gases Hydrogen, Nitrogen, Oxygen, Fluorine, Chlorine, Helium, Neon, Argon, Krypton, Xenon, and Radon All other elements are solids Distribution of Elements Elements are distributed unequally in nature Figure a, shows the distribution in the glalaxies Figure b, shows the distribution in Earths crust Figure c, shows the distribution in Humans Sources of Element Names Greek- • Iodine: from the Greek iodes meaning Color violet. Latin- • Fluorine: from the Latin fluere meaning Property to flow. The fluorine containing ore fluorospar is low melting. German- • Bismuth: from the German Color weisse mass which means white mass. Location • Germanium: discovered in 1866 by a German chemist. Famous- • Einsteinium: named for Albert Einstein. Scientists Symbols of the Elements Each element has an abbreviation. Iodine is taken from Greek work iodes, meaning violet. Bismuth is from German, weisse masse, white mass. Germanium is due to it’s discovery by a German Others are named in commemoration of famous scientist Symbols of the Elements Each element has an abbreviation – Symbols Some (14) have single letter The rest have 2 letters The symbol stands for the element itself For one atom of the element For a particular quantity of the element Rules. Symbols have either one or two letters If one letter – Capitalized If two letters – First letter capitalized – second letter lower case Rules governing symbols of the elements are: 1. Symbols have either one or two letters. 2. If one letter is used it is capitalized. H carbon C hydrogen 3. If two letters are used, only Ba Ne barium neon the first is capitalized. Symbols of the Elements Symbols and names are on the inside cover. Possibly make flash cards to learn these symbols. Metal, Nonmetals, and Metalloids Metal, Nonmetals, and Metalloids Metals Most of the elements are metals Solids at RT (except mercury) Malleable – Can be hammered or rolled into sheets. Ductile – Can be Drawn into wires High Melting point High density Combine with non-metals to form ionic compounds Often found as alloys – Homogenous mixtures Metal, Nonmetals, and Metalloids A few of the less reactive metals such as copper, silver and gold are found in the free state. Metals can mix with each other to form alloys. Brass is a mixture of copper and zinc. Bronze is a mixture of copper and tin. Steel is a mixture of carbon and iron. Metal, Nonmetals, and Metalloids Nonmetals Low melting points and density Generally poor conductors of heat and conductivity Combine with one another to form molecular compounds Metalloids Properties are intermediate between metals and nonmetals Some are raw material for semiconductor Metalloids boron silicon germanium arsenic antimony tellurium polonium Compounds Compound: A substance with a constant composition that can be broken down into elements by chemical processes. Atoms in a compound are always whole number ratios Two types - molecular and ionic Compounds Molecule – The smallest uncharged individual unit of a compound formed from 2 or more atoms Ion – Positively or negatively charged atom or group of atoms. Held together by attractive forces from positively and negatively charged ions Cation – Positively charged Anion – Negatively charged Compounds can be classified as molecular or ionic. Ionic compounds are held together by attractive forces between their positive and negative charges. Molecular compounds are held together by covalent bonds. Diatomic Molecules Special type of molecule Contain 2 atoms – alike or different Seven elements are diatomic molecules Hydrogen - H2 Oxygen - O2 Nitrogen - N2 Fluorine - F2 Chlorine - Cl2 Bromine - Br2 Iodine - I2 Need to know these!!!! Occurrence of Diatomic Molecules Hydrogen H Not found in nature. Found in nature. Hydrogen Nitrogen H2 N Not found in nature. Found in nature. Nitrogen N2 Chemical Formulas Used as abbreviations for compounds Shows the symbols and the ratio of the elements in a compound H2O Indicates 2 Hydrogens and one Oxygen H2SO4 Indicates 2 Hydrogens, 1 Sulfur, and 4 Oxygen Water has the formula H2O. • It does not contain free hydrogen, H2 or free oxygen, O2. • The H2 part of H2O means that 2 atoms of hydrogen are combined with one atom of oxygen in the water molecule. H2 O2 H2O chemical formulas Serve as abbreviations of the names of compounds. CaCl2 calcium chloride chemical formulas Tell which elements the compound is composed of and how many atoms of each element are present in a formula unit. CaCl2 calcium chlorine chemical formulas Show the symbols of the atoms of the elements present in a compound. CaCl2 Ca calcium Cl chlorine chemical formulas Show the ratio of the atoms of the elements present in a compound. CaCl2 2 Cl 1 Ca Chemical Formulas Formula of a compound contains the symbols of all the elements Formula contains one atom of an element the number 1 subscript is left out Formula contains more than one atom of the same element – the number is indicated as a subscript written to the right of the symbol of that atom When a formula contains more than one group of atoms occur as a unit – a parentheses is place around the group with the number subscripted Ca(NO3)2 Chemical Formulas H3PO4 indicates the element oxygen (O) indicates indicates the element indicates 4 O atoms hydrogen (H) 3 H atoms indicates the element phosphorous (P) Chemical Formulas indicates the element barium indicates two phosphate (PO 34 ) groups Ba3(PO4)2 indicates three Ba atoms indicates the phosphate group composed of one phosphorous atom and four oxygen atoms Chemical Formulas Formulas written as H2O, H2SO4, Ca(NO3)2 and C12H22O11 show only the number and kind of each atom contained in the compound; they do not show the arrangements of the atoms in the compound or how they are chemically bonded to each other. Concepts - Chapter 3 Classify – elements, compounds, mixtures Write symbols or name for common elements Understand chemical formulas Differentiate between atoms, molecules, ions Know some characteristics of metals, nonmetals and metalloids Recognize elements that occur as diatomic molecules