percent composition and formulas
... 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C2H6 + O2 ...
... 1. Write the correct formula(s) for the reactants on the left side and the correct formula(s) for the product(s) on the right side of the equation. Ethane reacts with oxygen to form carbon dioxide and water C2H6 + O2 ...
Web Supplement 7.2
... outlined, the following stepwise approach can be used. To illustrate, we return to the chromate example in which you are given the identities of the reactants and products and are told that the reaction proceeds under acidic conditions. Start with the skeleton reaction given in Eq. 7.W5. Step 1: Det ...
... outlined, the following stepwise approach can be used. To illustrate, we return to the chromate example in which you are given the identities of the reactants and products and are told that the reaction proceeds under acidic conditions. Start with the skeleton reaction given in Eq. 7.W5. Step 1: Det ...
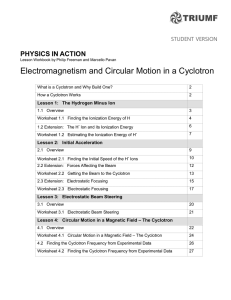
Worksheet 1.1 Finding the Ionization Energy of H
... What is a Cyclotron and Why Build One? The TRIUMF (the TRI University Meson Facility) cyclotron is one of a large class of particle accelerators. People used to call these “atom smashers” and that’s not a bad name (though these days a lot more than that is done with accelerators). One of the chief u ...
... What is a Cyclotron and Why Build One? The TRIUMF (the TRI University Meson Facility) cyclotron is one of a large class of particle accelerators. People used to call these “atom smashers” and that’s not a bad name (though these days a lot more than that is done with accelerators). One of the chief u ...
Chapter 8
... equation and on the product side of an equation. The top row in a chart gives the number and types of atoms on the reactant side and the bottom row gives the number and types of atoms on the product side of a chemical equation. Using a chart may make it easier to see where coefficients are needed in ...
... equation and on the product side of an equation. The top row in a chart gives the number and types of atoms on the reactant side and the bottom row gives the number and types of atoms on the product side of a chemical equation. Using a chart may make it easier to see where coefficients are needed in ...
Lecture 33 - Carbohydrate Metabolism 1
... tripeptide that has a free sulfhydryl group which functions as an electron donor in a variety of coupled redox reactions in the cell. ...
... tripeptide that has a free sulfhydryl group which functions as an electron donor in a variety of coupled redox reactions in the cell. ...
chap-4-atomic-weights
... The problem with using the above method to determine the relative weights of atoms was that there was no way to tell if water was really HO or H2O or HO2. Dalton claimed that the tendency of things to vaporize probably meant that atoms repelled each other - so no more would stick together than were ...
... The problem with using the above method to determine the relative weights of atoms was that there was no way to tell if water was really HO or H2O or HO2. Dalton claimed that the tendency of things to vaporize probably meant that atoms repelled each other - so no more would stick together than were ...
Chemical Equations
... Note, there appear to be more oxygen atoms, fewer hydrogen atoms at the end that at the beginning! ...
... Note, there appear to be more oxygen atoms, fewer hydrogen atoms at the end that at the beginning! ...
Chapter #3
... Formula Units in a given Mass of Compound Problem 7-3: Trisodium phosphate is a component of some detergents. How many moles and formula units are in a 38.6 g sample? Plan: We need to determine the formula, and the molecular mass from the atomic masses of each element multiplied by the coefficients. ...
... Formula Units in a given Mass of Compound Problem 7-3: Trisodium phosphate is a component of some detergents. How many moles and formula units are in a 38.6 g sample? Plan: We need to determine the formula, and the molecular mass from the atomic masses of each element multiplied by the coefficients. ...
Reaction Stoichiometry
... Chlorobenzene, C6H5Cl, is used in the production of many important chemicals, such as aspirin, dyes, and disinfectants. One industrial method of preparing chlorobenzene is to react benzene, C6H6, with chlorine. C6H6(l) + Cl2(g) → C6H5Cl(l) + HCl(g) When 36.8 g benzene react with an excess of Cl2, th ...
... Chlorobenzene, C6H5Cl, is used in the production of many important chemicals, such as aspirin, dyes, and disinfectants. One industrial method of preparing chlorobenzene is to react benzene, C6H6, with chlorine. C6H6(l) + Cl2(g) → C6H5Cl(l) + HCl(g) When 36.8 g benzene react with an excess of Cl2, th ...
Document
... Check for Understanding Aqueous potassium nitrate and a precipitate of barium chromate are formed when aqueous solutions of barium nitrate and potassium chromate are mixed. Ba(NO3)2 (aq) + K2CrO4 (aq) ...
... Check for Understanding Aqueous potassium nitrate and a precipitate of barium chromate are formed when aqueous solutions of barium nitrate and potassium chromate are mixed. Ba(NO3)2 (aq) + K2CrO4 (aq) ...
Slide 1
... Some are stable Some are not... they 'decay' - lose the neutron These 'radioisotopes' emit energy (radiation) This process is not affected by environmental conditions and is constant; so if we know the amount of parent and daughter isotope, and we know the decay rate, we can calculate the time it ha ...
... Some are stable Some are not... they 'decay' - lose the neutron These 'radioisotopes' emit energy (radiation) This process is not affected by environmental conditions and is constant; so if we know the amount of parent and daughter isotope, and we know the decay rate, we can calculate the time it ha ...
1. Metabolic pathways 2. Basic enzyme kinetics 3. Metabolic
... » Electrons are transported from NADH & FADH through the electron transport chain to oxygen » Electron transport causes protons to be released into the intermembrane space » These electrons can be transported back into mitochondrial matrix by a proton conducting ATP-synthase » The detailed mechanist ...
... » Electrons are transported from NADH & FADH through the electron transport chain to oxygen » Electron transport causes protons to be released into the intermembrane space » These electrons can be transported back into mitochondrial matrix by a proton conducting ATP-synthase » The detailed mechanist ...
RSC_QTECR_ch005 105..131
... and quantum tunnelling are significant in determining free-energy reaction barriers.2,3 The incorporation of nuclear quantum effects (NQE) is also important for reactions involving heavy atoms since one of the most direct experimental assessment of the transition state and the mechanism of a chemical ...
... and quantum tunnelling are significant in determining free-energy reaction barriers.2,3 The incorporation of nuclear quantum effects (NQE) is also important for reactions involving heavy atoms since one of the most direct experimental assessment of the transition state and the mechanism of a chemical ...
The Mole
... 4. How can you calculate the mass of a mole of a compound? 5. How many moles is 1.50 x 1023 molecules of ...
... 4. How can you calculate the mass of a mole of a compound? 5. How many moles is 1.50 x 1023 molecules of ...
Experimental determination of hydromagnesite precipitation rates
... by the continual polymerization of monosilicic acid (H4SiO4). These aggregated structures had a total specific surface area of ~ 0.24 m2/g. Interestingly, nuclear magnetic resonance measurements revealed a low overall porosity of ~ 8%, but with a high proportion of pores in the nm-range, and only ~ ...
... by the continual polymerization of monosilicic acid (H4SiO4). These aggregated structures had a total specific surface area of ~ 0.24 m2/g. Interestingly, nuclear magnetic resonance measurements revealed a low overall porosity of ~ 8%, but with a high proportion of pores in the nm-range, and only ~ ...
notes - unit 2 - atomic theory_student_2012
... 1. Give the correct electron configuration for an atom of oxygen. _______________ 2. Give the correct electron configuration for the K+ ion. _______________ 3. How many valence electrons are there in atom of fluorine? ________ 4. How many valence electrons are there in an F- ion? ________ 5. How man ...
... 1. Give the correct electron configuration for an atom of oxygen. _______________ 2. Give the correct electron configuration for the K+ ion. _______________ 3. How many valence electrons are there in atom of fluorine? ________ 4. How many valence electrons are there in an F- ion? ________ 5. How man ...
hty utI! rn h 1m 0 nt - Northside Middle School
... The acceptance of atomic theory was only the beginning of our understanding of matter. Once scientists were fairly convinced of the existence of atoms, the next set of questions to be answered emerged. What is an atom like? How are atoms shaped? Is the composition of an atom uniform throughout, or i ...
... The acceptance of atomic theory was only the beginning of our understanding of matter. Once scientists were fairly convinced of the existence of atoms, the next set of questions to be answered emerged. What is an atom like? How are atoms shaped? Is the composition of an atom uniform throughout, or i ...
chem pre ap atom and nuclear practice test
... c. uranium-235 absorbing a neutron and breaking into barium-141, krypton-92, and three neutrons d. a glucose molecule being metabolized with oxygen to form carbon dioxide and water ____ 11. Scientists are investigating the possibility of containing fusion reactions within a. steel containers. c. con ...
... c. uranium-235 absorbing a neutron and breaking into barium-141, krypton-92, and three neutrons d. a glucose molecule being metabolized with oxygen to form carbon dioxide and water ____ 11. Scientists are investigating the possibility of containing fusion reactions within a. steel containers. c. con ...
4.Lect Carbon skeleton intro
... synthesize glucose and are termed glucogenic. while some are converted to acetylCoA (ketogenic amino acids) these CANNOT be used to synthesize glucose. Ketogenic amino acids can be converted to fatty acids for storage as triglyceride and later oxidation (fed state), or to ketone bodies (made in live ...
... synthesize glucose and are termed glucogenic. while some are converted to acetylCoA (ketogenic amino acids) these CANNOT be used to synthesize glucose. Ketogenic amino acids can be converted to fatty acids for storage as triglyceride and later oxidation (fed state), or to ketone bodies (made in live ...
Chemistry: A Molecular Approach
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
... Dalton proposed a theory of matter based on it having ultimate, indivisible particles to explain these laws Each element is composed of tiny, indestructible particles called atoms All atoms of a given element has the same mass and other properties that distinguish them from atoms of other elements A ...
Types of Chemical Reactions (rxns.)
... 2. Predict the product(s) using the type of reaction as a ...
... 2. Predict the product(s) using the type of reaction as a ...
Stage 2 Chemistry Intended Student Learning 2014
... This topic deals with some of the underlying principles of chemistry (‘elemental chemistry’) and then considers the chemistry of the environment. The elemental chemistry component of the topic focuses on the periodic table and the concept of electronegativity; together these underlie most of the oth ...
... This topic deals with some of the underlying principles of chemistry (‘elemental chemistry’) and then considers the chemistry of the environment. The elemental chemistry component of the topic focuses on the periodic table and the concept of electronegativity; together these underlie most of the oth ...
METABOLISM IN HEALTH AND DISEASES I Lecture 2 Pentose
... the pentose phosphate pathway. • G6PD converts glucose-6-phosphate into 6-phosphogluconoδ-lactone • This is the rate-limiting enzyme of this metabolic pathway that supplies reducing energy to cells by maintaining the level of the co-enzyme nicotinamide adenine dinucleotide phosphate(NADPH) • The NAD ...
... the pentose phosphate pathway. • G6PD converts glucose-6-phosphate into 6-phosphogluconoδ-lactone • This is the rate-limiting enzyme of this metabolic pathway that supplies reducing energy to cells by maintaining the level of the co-enzyme nicotinamide adenine dinucleotide phosphate(NADPH) • The NAD ...
Using Chemical Formulas Power ponit
... Example: Find the molar mass for KClO3. KClO3 has 1 potassium (K) atom, 1 chlorine atom (Cl), and 3 oxygen (O) atoms. Atomic mass of potassium = 39.10 amu Atomic mass of chlorine = 35.45 amu Atomic mass of oxygen = 16.00 amu 1 potassium atom x 39.10 amu = 39.10 amu ...
... Example: Find the molar mass for KClO3. KClO3 has 1 potassium (K) atom, 1 chlorine atom (Cl), and 3 oxygen (O) atoms. Atomic mass of potassium = 39.10 amu Atomic mass of chlorine = 35.45 amu Atomic mass of oxygen = 16.00 amu 1 potassium atom x 39.10 amu = 39.10 amu ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.