The road to knowledge: from biology to databases and back again
... HumanCyc enables selecting, for instance, only small molecule reactions. The exact representation of a metabolic reaction differs per database. For example, each database uses a different terminology in its data model to refer to the metabolites before the arrow, e.g., ‘substrates’ or ‘input’, and a ...
... HumanCyc enables selecting, for instance, only small molecule reactions. The exact representation of a metabolic reaction differs per database. For example, each database uses a different terminology in its data model to refer to the metabolites before the arrow, e.g., ‘substrates’ or ‘input’, and a ...
Chemical Compounds
... 4. The oxidation state of hydrogen is generally +1 except when it is bonded to metals such as sodium (NaH) in which case it's oxidation number is -1. 5. Fluorine has an oxidation number of -1 in its compounds … always. Group 1 elements have an oxidation number of +1 in their compounds … always. Grou ...
... 4. The oxidation state of hydrogen is generally +1 except when it is bonded to metals such as sodium (NaH) in which case it's oxidation number is -1. 5. Fluorine has an oxidation number of -1 in its compounds … always. Group 1 elements have an oxidation number of +1 in their compounds … always. Grou ...
chapter 3 Questions
... species to attract the males for mating. One pheromone has the molecular formula C19H38O. Normally, the amount of this pheromone secreted by a female insect is about 1.0 10-12 g. How many molecules are there in this quantity? A) 1.0 1012 B) 6.0 1011 C) 2.3 1010 D) 2.1 109 ...
... species to attract the males for mating. One pheromone has the molecular formula C19H38O. Normally, the amount of this pheromone secreted by a female insect is about 1.0 10-12 g. How many molecules are there in this quantity? A) 1.0 1012 B) 6.0 1011 C) 2.3 1010 D) 2.1 109 ...
Stereochemical imperative in enzymic decarboxylations
... containing Br2 and NaOD. The acetic acid formed was purified as described above and analyzed by mass spectroscopy. Mass Spectroscopy. Specimens (1-5 mg) of labeled sodium acetate prepared as described above were acidified with 50 p L of 85% phosphoric acid. The samples were then evacuated briefly to ...
... containing Br2 and NaOD. The acetic acid formed was purified as described above and analyzed by mass spectroscopy. Mass Spectroscopy. Specimens (1-5 mg) of labeled sodium acetate prepared as described above were acidified with 50 p L of 85% phosphoric acid. The samples were then evacuated briefly to ...
Phosphoketolase pathway dominates in
... were harvested when high extracellular glucose levels still remained in the media. A duplicate sample from a different culture gave identical results. Growth could be greatly improved in the presence of fructose (Table 2), and the cells contained higher levels of ATP (Fig. 2C). L. reuteri ATCC 55730 ...
... were harvested when high extracellular glucose levels still remained in the media. A duplicate sample from a different culture gave identical results. Growth could be greatly improved in the presence of fructose (Table 2), and the cells contained higher levels of ATP (Fig. 2C). L. reuteri ATCC 55730 ...
Moles - tamchemistryhart
... can’t see them? How can we count how many atoms or molecules are in a piece of matter if they have different masses? ...
... can’t see them? How can we count how many atoms or molecules are in a piece of matter if they have different masses? ...
CHEMICAL EQUATIONS - Clayton State University
... States of reactants and products Physical states of reactants and products are represented by: (g): gas (l): liquid (s): solid (aq): aqueous or water solution ...
... States of reactants and products Physical states of reactants and products are represented by: (g): gas (l): liquid (s): solid (aq): aqueous or water solution ...
Magic-angle Spinning Nuclear Magnetic Resonance Studies of
... with water physically adsorbed in the zeolite cavities. Bronnimann et aL4 found three narrow lines in the 'H MAS NMR spectra of weakly hydrated aluminosilicates and assigned the signal at 7.0 ppm to hydrated Brernsted-acid sites. Hence a bare Brernsted-acid site should have a much higher chemical sh ...
... with water physically adsorbed in the zeolite cavities. Bronnimann et aL4 found three narrow lines in the 'H MAS NMR spectra of weakly hydrated aluminosilicates and assigned the signal at 7.0 ppm to hydrated Brernsted-acid sites. Hence a bare Brernsted-acid site should have a much higher chemical sh ...
Print this article - Journals at the University of Arizona
... well as terrestrial carbohydrates are being consumed, NEAAs are thought more likely to contain proportionally higher 14C content than the more depleted reservoir 14C found in EAAs. Under such circumstances, we would expect to find a difference in 14C dates of EAAs and NEAAs from the same individual. ...
... well as terrestrial carbohydrates are being consumed, NEAAs are thought more likely to contain proportionally higher 14C content than the more depleted reservoir 14C found in EAAs. Under such circumstances, we would expect to find a difference in 14C dates of EAAs and NEAAs from the same individual. ...
chemistry -- questions -
... __ 25. A research article indicates that researchers have used an isotope 3H to trace a certain metabolic process. 2H contains one protein, therefore, from the symbol that is given, we know this is a hydrogen isotope with a) three protons. b) three neutrons. c) three electrons. d) one proton and two ...
... __ 25. A research article indicates that researchers have used an isotope 3H to trace a certain metabolic process. 2H contains one protein, therefore, from the symbol that is given, we know this is a hydrogen isotope with a) three protons. b) three neutrons. c) three electrons. d) one proton and two ...
CHEM-1411 Final Practice Exam
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
1411FINALSAMPLE+KEY - Houston Community College
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
... sulfur atom in the first structure is therefore sp3. However, the sulfur is not simply sp3 hybridized in the second structure, which has an “expanded octet” around the sulfur atom. Hybridizations that allow more than an octet of electrons around an atom are sp3d (10 electrons) and sp3d2 (12 electron ...
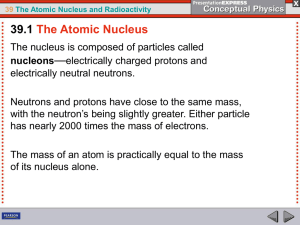
39 The Atomic Nucleus and Radioactivity
... New Zealander Ernest Rutherford, in 1919, was the first physicist to succeed in artificially transmuting a chemical element. He bombarded nitrogen nuclei with alpha particles and found traces of oxygen and hydrogen that were not there before. Rutherford accounted for the presence of the oxygen and h ...
... New Zealander Ernest Rutherford, in 1919, was the first physicist to succeed in artificially transmuting a chemical element. He bombarded nitrogen nuclei with alpha particles and found traces of oxygen and hydrogen that were not there before. Rutherford accounted for the presence of the oxygen and h ...
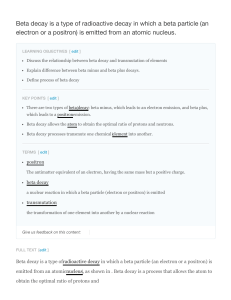
Beta decay is a type of radioactive decay in which a beta
... Since the proton and neutron are part of an atomic nucleus, beta decay processes result in transmutation of one chemical element into another. For example: 137Cs ...
... Since the proton and neutron are part of an atomic nucleus, beta decay processes result in transmutation of one chemical element into another. For example: 137Cs ...
Chapter 3 Chemical Reactions and Reaction Stoichiometry
... the production of alcoholic beverages like beer and wine. Chemical reactions that produce CO2 are not limited to cooking, though—they occur in places as diverse as the cells in your body and the engine of your car. © 2014 Pearson Education, Inc. ...
... the production of alcoholic beverages like beer and wine. Chemical reactions that produce CO2 are not limited to cooking, though—they occur in places as diverse as the cells in your body and the engine of your car. © 2014 Pearson Education, Inc. ...
1 - Grygla School
... quantity of matter in an object. As long as the object is not changed, it will have the same mass, no matter where it is in the universe. On the other hand, the weight of that object is affected by its location in the universe. The weight depends on gravity, while mass does not. Weight is defined as ...
... quantity of matter in an object. As long as the object is not changed, it will have the same mass, no matter where it is in the universe. On the other hand, the weight of that object is affected by its location in the universe. The weight depends on gravity, while mass does not. Weight is defined as ...
New Insight into the Role of the Calvin Cycle: Reutilization
... the CBB cycle in the heterotrophic P(3HB) biosynthesis from sugars. We assumed that, when the CBB cycle is functional under heterotrophic conditions in the presence of sugars, it may act on fixation and reutilization of CO2 emitted by oxidative decarboxylation during the sugar degradation. Generally ...
... the CBB cycle in the heterotrophic P(3HB) biosynthesis from sugars. We assumed that, when the CBB cycle is functional under heterotrophic conditions in the presence of sugars, it may act on fixation and reutilization of CO2 emitted by oxidative decarboxylation during the sugar degradation. Generally ...
Substitution reactions of coordinated P-diketones p
... synthetic aspect assumes i>:nportance in such readily available materials as the metal /3dicarbonyl chelates (and related compounds) where substitution of reactive functional groups for the H atom attached directly to the ring would give rise to polyfunctional chelates. These in addition to any othe ...
... synthetic aspect assumes i>:nportance in such readily available materials as the metal /3dicarbonyl chelates (and related compounds) where substitution of reactive functional groups for the H atom attached directly to the ring would give rise to polyfunctional chelates. These in addition to any othe ...
Appendix N CONCENTRATION UNITS
... Parts per million is used to describe concentrations in solutions containing poorly defined or unidentified solutes or mixtures of solutes. Parts per million is also used to describe concentration levels to those who are unfamiliar with the concept of moles. Tap water contains a variety of dissolved ...
... Parts per million is used to describe concentrations in solutions containing poorly defined or unidentified solutes or mixtures of solutes. Parts per million is also used to describe concentration levels to those who are unfamiliar with the concept of moles. Tap water contains a variety of dissolved ...
Engineering primary metabolic pathways of industrial
... in the network. Assuming the metabolite concentrations are in steady state, a set of algebraic equations relating the fluxes is obtained. Linear programming is then used to calculate the fluxes through the branches of the network (Ren et al., 2003; Stephanopoulos et al., 1998). Further information i ...
... in the network. Assuming the metabolite concentrations are in steady state, a set of algebraic equations relating the fluxes is obtained. Linear programming is then used to calculate the fluxes through the branches of the network (Ren et al., 2003; Stephanopoulos et al., 1998). Further information i ...
The mole and calculations
... How do empirical formulas tie into molecular formulas?? They each contain the same atoms (e.g. if we have an empirical formula of CH, the molecular formula will also have CH in it - and nothing else!!) but the difference is, molecular formulas are some whole number multiple of the empirical formul ...
... How do empirical formulas tie into molecular formulas?? They each contain the same atoms (e.g. if we have an empirical formula of CH, the molecular formula will also have CH in it - and nothing else!!) but the difference is, molecular formulas are some whole number multiple of the empirical formul ...
CH 151 Companion
... Safety is of utmost importance. Work in the laboratory should be a safe experience. It will be safe, however, only if certain safety precautions are followed without exception. Safety is up to you. Everyone working in the chemistry laboratories must follow the following rules. Your instructor will d ...
... Safety is of utmost importance. Work in the laboratory should be a safe experience. It will be safe, however, only if certain safety precautions are followed without exception. Safety is up to you. Everyone working in the chemistry laboratories must follow the following rules. Your instructor will d ...
molecular formula
... atoms of each element present in the compound. The molecular formula represents the total number of atoms of each element present in one molecule of a compound. ...
... atoms of each element present in the compound. The molecular formula represents the total number of atoms of each element present in one molecule of a compound. ...
- Te Kura
... This topic consists of 10 lessons covering the fundamental concepts of curriculum level 7 chemistry. It is recommended that you complete this booklet to revise these concepts. If you feel confident that you have understood the concepts of a lesson, you can skip the activities. You are expected to co ...
... This topic consists of 10 lessons covering the fundamental concepts of curriculum level 7 chemistry. It is recommended that you complete this booklet to revise these concepts. If you feel confident that you have understood the concepts of a lesson, you can skip the activities. You are expected to co ...
J. Am. Chem. SOC. 1993,115, 7685-7695
... A similar reaction occurs with the symmetric arene 2,6dimethoxynaphthalene. The single product shows bound 72resonances at 6 3.023 (td, J = 7.6, 1.9 Hz) and 3.664 (td, J = 7.8, 2.4 Hz) and a 31Pdoublet at 6 0.50 ( J = 201 Hz), as well as two distinct methoxy resonances. The formation of the 3,4-q2-2 ...
... A similar reaction occurs with the symmetric arene 2,6dimethoxynaphthalene. The single product shows bound 72resonances at 6 3.023 (td, J = 7.6, 1.9 Hz) and 3.664 (td, J = 7.8, 2.4 Hz) and a 31Pdoublet at 6 0.50 ( J = 201 Hz), as well as two distinct methoxy resonances. The formation of the 3,4-q2-2 ...
Isotopic labeling

Isotopic labeling (or isotopic labelling) is a technique used to track the passage of an isotope, or an atom with a variation, through a reaction, metabolic pathway, or cell. The reactant is 'labeled' by replacing specific atoms by their isotope. The reactant is then allowed to undergo the reaction. The position of the isotopes in the products is measured to determine the sequence the isotopic atom followed in the reaction or the cell's metabolic pathway. The nuclides used in isotopic labeling may be stable nuclides or radionuclides. In the latter case, the labeling is called radiolabeling.In isotopic labeling, there are multiple ways to detect the presence of labeling isotopes; through their mass, vibrational mode, or radioactive decay. Mass spectrometry detects the difference in an isotope's mass, while infrared spectroscopy detects the difference in the isotope's vibrational modes. Nuclear magnetic resonance detects atoms with different gyromagnetic ratios. The radioactive decay can be detected through an ionization chamber or autoradiographs of gels.An example of the use of isotopic labeling is the study of phenol (C6H5OH) in water by replacing common hydrogen (protium) with deuterium (deuterium labeling). Upon adding phenol to deuterated water (water containing D2O in addition to the usual H2O), the substitution of deuterium for the hydrogen is observed in phenol's hydroxyl group (resulting in C6H5OD), indicating that phenol readily undergoes hydrogen-exchange reactions with water. Only the hydroxyl group was affected, indicating that the other 5 hydrogen atoms did not participate in these exchange reactions.